Abstract
Present study investigated the morphologic changes of autonomic nerves in the adipose tissue in diabetic animal model. Male obese type 2 diabetic db/db mice and age matched non-diabetic db/m control mice were used. Epididymal adipose tissue from diabetic db/db mice with that from control heterozygous db/m mice was compared using confocal microscopy-based method to visualize intact whole adipose tissue. Immunohistochemistry with tyrosine hydroxylase for sympathetic (SP), choline acetyltransferase for parasympathetic (PSP), and protein gene product 9.5 (PGP 9.5) for whole autonomic nerves was performed. The quantity of immunostained portion of SP, PSP, and PGP 9.5 stained nerve fibers showed decreased trend in diabetic group; however, the ratio of SP/PSP of adipose tissue was higher in diabetic group compared with control group as follows (0.70±0.30 vs. 0.95±0.25, P<0.05; normal vs. diabetic, respectively). Both SP and PSP nerve fibers were observed in white adipose tissue and PSP nerve fibers were suggested as more decreased in diabetes based on our observation.
Adipose tissue is composed of adipocytes, periadipocytes, fibrocytes, vascular endothelial cells, and immune cells [1]. Neurons have been postulated to play an important role in the regulation of adipose tissue. With respect to lipid mobilization, white adipose tissue (WAT) is innervated by sympathetic (SP) peripheral nerves; lipid metabolism is also related to peripheral nerve regeneration. On the other hand, autonomic neuropathy involving SP and parasympathetic (PSP) nerves is a common chronic complication in diabetes. Autonomic nerve fibers are prone to damage depending on their location and length. This damage is a subtype of diabetic neuropathy, which can also occur in adipose tissue in relation with autonomic neuropathy or peripheral neuropathy [23], similar to enteric organs in the human body. In obese patients, visceral WAT is associated with increased risk of type 2 diabetes mellitus; however, some patients exhibit decreased fat mass as diabetes progresses although cardiac autonomic neuropathy can be associated with higher waist circumference [4]. In the later stages of diabetes progression, neuronal regulation of adipose tissue may be different from that in the initial period of diabetes diagnosis. However, the relationship between neuronal changes and diabetes progression has not yet been studied. In particular, no studies have focused on the relationship between innervation state and neuronal regulation in adipose tissue. Therefore, it is important to clarify the complex relationship between adipose tissue and autonomic nerves. Therefore, we investigated the neuronal morphology changes in adipose tissue in db/db diabetic mice and compared them with those in normal glucose-metabolizing mice.
All animal work was approved by the Institutional Animal Care and Use of Chonbuk National University Hospital (Approval number: cuh-IACUC 2017-8). For this study, db/db mice with similar weights and blood glucose concentrations were assigned to the diabetic group and db/− mice showing normal glucose values were assigned to the control group. Blood glucose levels were measured from the tail vein every week after 8 hours of fasting using a test strip and a Precision Xtra Plus® meter (Abbot Laboratories, MediSence Products, Bedford, MA, USA). Mice with blood glucose levels greater than 350 mg/dL were considered to be diabetic. A commercially available kit (NycoCard, Oslo, Norway) was used to measure glycosylated hemoglobin (HbA1c) levels. All mice were sacrificed and peripheral nerve morphologic changes in adipose tissue were compared between the normal and diabetic groups. Adipose tissue was obtained from mouse epididymal fat at 8 weeks and stained for innervated nerve fibers using protein gene product 9.5 (PGP 9.5). Tyrosine hydroxylase (TH) and choline acetyltransferase (ChAT) were used to detect SP and PSP nerve fibers, respectively. Next, neurons were measured quantitatively by determining the total innervated length of each SP and PSP nerve fiber. The values were compared between the normal and diabetic groups. After measuring the total length of each SP and PSP nerve fiber, the ratios of SP/PSP nerve fibers were compared between the normal and diabetic groups. All data are expressed as mean±standard deviation. Data were analyzed for statistically significant differences between the two groups using the t-test. Statistical significance was defined as P<0.05 with a 95% confidence interval. Statistical analysis was performed using SPSS version 18.0 software (SPSS Inc., Chicago, IL, USA).
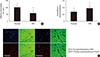
The mean weights of the normal and diabetic groups were significantly different (55.8±2.1 g vs. 50.8±1.8 g, respectively; P< 0.05). Moreover, the mean blood glucose value was significantly higher in the diabetic group compared to the normal group (432±42 mg/dL vs. 98±10 mg/dL, respectively; P<0.05). HbA1c levels showed a similar trend as blood glucose values (4.8%±0.4% vs. 12.2%±2.8%, normal vs. diabetic, respectively; P<0.05). Adipocytes were smaller in diabetic mice, although the numbers of adipocytes were not assessed. Less innervated adipose tissue, as assessed by immunostaining with PGP 9.5, was observed in diabetic mice compared with normal glucose-metabolizing mice. This assessment was performed by measuring the occupied neuronal areas (100,806.4±29,323 µm2 vs. 58,457.5±38,323 µm2, normal vs. diabetes, respectively; P<0.05). The area of TH-stained SP nerve fibers was reduced less than that of ChAT-stained PSP nerve fibers in the diabetic group; thus, the SP/PSP ratio was higher in the diabetic group than in the normal group (0.7±0.3 vs. 0.95±0.25, normal vs. diabetic, respectively; P<0.05) (Fig. 1).
Although adipose tissue has been traditionally thought of as an innervated endocrine gland, research has also focused on its role in metabolic disorders. Metabolic disorders have also been thought to be due to problematic peripheral nerve distribution in adipose tissue, although the roles of SP and PSP activity and the importance of their balance in adipose tissue have also been considered [56]. While peripheral nerves in adipose tissue are branches of autonomic nerve fibers, these fibers can also be classified as metabolically implicated nerve fibers or as anatomically distributed nerves without metabolic function. However, metabolically important sensory neurons containing transient receptor potential channels are also distributed in adipose tissue and play a role in energy balance [7].
Adipocytes contain both afferent and efferent peripheral nerves, which can communicate with the brain via diverse signals, including leptin and adipokines. However, the precise pathways of communication have not yet been identified [8]. Adipose tissue can be classified according to its location and function, e.g., visceral fat versus subcutaneous fat and WAT versus brown adipose tissue (BAT). Among these types, excess visceral adipose tissue has been shown to be important in obesity-related diseases and in diabetes [91011]. Currently, little is known regarding the roles of peripheral nerves in adipose tissue and their changes in diabetes with respect to metabolic outcomes or peripheral neuropathies. SP innervation and sensory nerves are included in BAT and WAT adipose tissue with vasculature innervation. In WAT, sensory innervation has been identified by immunohistochemical labeling of neuronal peptides such as substance P or calcitonin gene-related peptide [8]. SP innervation of the autonomic nerve system was demonstrated before its neuroanatomical and physiological roles in adipose tissue were shown [1213]. Another study investigated PSP input and its functional implications in adipose tissue in the context of insulin sensitivity and glucose metabolism [14]. However, there is no clear cause-and-effect relationship between adipose tissue and innervating peripheral nerves in diabetes, especially because no studies have focused on the relationships between diabetic neuropathy and fat mass or between diabetic neuropathy and changes of adipose tissue function. Similar to the diverse range of sensory neuropathies, sensory neuronal function in adipose tissue can also deteriorate. This deterioration may influence adipocyte structure or function via the adipocyte-brain conduit, if present. In addition, complications such as diabetic neuropathy or neuronal degeneration can also harm adipose tissue function in diabetes.
We observed fewer peripheral nerves in adipose tissue in diabetes; moreover, quantitative analysis revealed that PSP nerves were more prone to damage. Future studies should aim to identify the order in which adipose tissue and peripheral nerve fibers are affected by the risk factors of diabetic peripheral neuropathy (DPN) in diabetes. Moreover, in this study, small nerve fibers located in adipose tissue displayed more damage under diabetic conditions. However, the clinical significance of this neural damage was not determined. Therefore, it is unknown whether this damage affects adipokine regulation or whether it reflects deteriorated neuronal status in other tissue.
In summary, it has not been clear whether adipose tissue changes as diabetes complications develop and DPN progresses. It has also been unclear whether DPN aggravation leads to adipose tissue degeneration. Moreover, besides central regulation, it is not clear whether neural regulation via the autonomic nerve system is related to adipose tissue, autonomic nerves in adipose tissue, and DPN. Therefore, further studies aiming to determine the exact functional and morphological associations between autonomic nerves, somatic sensory nerves, and adipose tissue innervation in diabetes are warranted.
Figures and Tables
Fig. 1
Quantitative comparison of peripheral nerves in adipose tissue from db/db diabetic mice versus those from db/− control mice. (A) Quantity of PGP 9.5 (protein gene product 9.5)-positive immunostained nerve fibers, expressed as the total nerve fiber area. (B) Ratios of the occupied areas of sympathetic and parasympathetic nerve fibers. (C) Tyrosine hydroxylase (TH; red) and choline acetyltransferase (ChAT; blue) immunohistochemistry staining results of sympathetic and parasympathetic nerve fibers, respectively. Data are presented as mean±standard deviation (n=10 per group). DM, diabetes mellitus. aP<0.05 vs. the normal group.
ACKNOWLEDGMENTS
This paper was partly supported by the fund of Clinical Medicine Research Institute of Chonbuk National University-Biomedical Research Institute of Chonbuk National University Hospital.
References
1. Kershaw EE, Flier JS. Adipose tissue as an endocrine organ. J Clin Endocrinol Metab. 2004; 89:2548–2556.

2. Jang EH, Kim NY, Park YM, Kim MK, Baek KH, Song KH, Lee KW, Kwon HS. Influence of visceral adiposity on cardiovascular autonomic neuropathy in patients with type 2 diabetes mellitus. Diabetes Metab J. 2012; 36:285–292.

3. Bittel DC, Bittel AJ, Tuttle LJ, Hastings MK, Commean PK, Mueller MJ, Cade WT, Sinacore DR. Adipose tissue content, muscle performance and physical function in obese adults with type 2 diabetes mellitus and peripheral neuropathy. J Diabetes Complications. 2015; 29:250–257.

4. Voulgari C, Psallas M, Kokkinos A, Argiana V, Katsilambros N, Tentolouris N. The association between cardiac autonomic neuropathy with metabolic and other factors in subjects with type 1 and type 2 diabetes. J Diabetes Complications. 2011; 25:159–167.

5. Fliers E, Romijn JA, Sauerwein HP, Kalsbeek A, Kreier F, Buijs RM. Adipose tissue: an innervated endocrine gland. Ned Tijdschr Geneeskd. 2002; 146:1976–1979.
6. Romijn JA, Fliers E. Sympathetic and parasympathetic innervation of adipose tissue: metabolic implications. Curr Opin Clin Nutr Metab Care. 2005; 8:440–444.

7. Blaszkiewicz M, Townsend KL. Adipose tissue and energy expenditure: central and peripheral neural activation pathways. Curr Obes Rep. 2016; 5:241–250.

8. Bartness TJ, Liu Y, Shrestha YB, Ryu V. Neural innervation of white adipose tissue and the control of lipolysis. Front Neuroendocrinol. 2014; 35:473–493.

9. Kern PA, Ranganathan S, Li C, Wood L, Ranganathan G. Adipose tissue tumor necrosis factor and interleukin-6 expression in human obesity and insulin resistance. Am J Physiol Endocrinol Metab. 2001; 280:E745–E751.

10. Marette A. Molecular mechanisms of inflammation in obesity-linked insulin resistance. Int J Obes Relat Metab Disord. 2003; 27:Suppl 3. S46–S48.

11. Mokdad AH, Ford ES, Bowman BA, Dietz WH, Vinicor F, Bales VS, Marks JS. Prevalence of obesity, diabetes, and obesity-related health risk factors, 2001. JAMA. 2003; 289:76–79.

12. Bamshad M, Aoki VT, Adkison MG, Warren WS, Bartness TJ. Central nervous system origins of the sympathetic nervous system outflow to white adipose tissue. Am J Physiol. 1998; 275(1 Pt 2):R291–R299.
13. Penicaud L, Cousin B, Leloup C, Lorsignol A, Casteilla L. The autonomic nervous system, adipose tissue plasticity, and energy balance. Nutrition. 2000; 16:903–908.

14. Kreier F, Fliers E, Voshol PJ, Van Eden CG, Havekes LM, Kalsbeek A, Van Heijningen CL, Sluiter AA, Mettenleiter TC, Romijn JA, Sauerwein HP, Buijs RM. Selective parasympathetic innervation of subcutaneous and intra-abdominal fat--functional implications. J Clin Invest. 2002; 110:1243–1250.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download