Abstract
Over the past three decades, human pancreatic islet isolation and transplantation techniques have developed as a routine clinical procedure for selected patients with type 1 diabetes mellitus. However, due to the donor shortage and required chronic systemic immunosuppression, the widespread application of islet transplantation is limited. To overcome these limitations, providing a physical barrier to transplanted islet cells with encapsulating biomaterial has emerged as a promising approach to enhance engraftment and promote islet survival post-transplantation. Alginate has been considered to be a reliable biomaterial, as it enhances islet survival and does not hamper hormone secretion. Alginate-catechol (Al-CA) hydrogel was reported to provide high mechanical strength and chemical stability without deformation over a wide range of pH values. In this study, we, demonstrated, for the first time in the literature, that encapsulation of murine pancreatic islet cells with Al-CA hydrogel does not induce cytotoxicity ex vivo for an extended period; however, it does markedly abate glucose-stimulated insulin secretion. Catechol should not be considered as a constituent for alginate gelation for encapsulating islet cells in the application of islet transplantation.
Type 1 diabetes mellitus (T1DM) is an autoimmune disease characterized by destruction of insulin-producing pancreatic β-cells, leading to insulin deficiency [1]. It represents 5% to 10% of the diagnosed cases of diabetes, corresponding to approximately 20 million cases worldwide as of 2014 [2]. The most common therapeutic modality for T1DM is the exogenous insulin injection; however, it is an ongoing clinical challenge as injection-related discomfort often leads to poor adherence to the therapy, and, consequently, hyperglycemia-associated complications in patients with T1DM. Recently, islet transplantation has been considered to be an effective therapy to normalize glycemic control in patients with T1DM [3]. However, the limited supply of pancreas and the need for life-long immunosuppression restrict its widespread application [4]. To overcome these drawbacks, providing a physical barrier to the transplanted islet cells by encapsulation to achieve biocompatibility and immunoisolation has emerged as a promising approach [5]. Although there is no consensus on the best material for islet encapsulation, the best reported material for survival in rodent studies was alginate [6]. Moreover, it was reported that alginate does not interfere with the islet functions in releasing hormone [7]. A recent study reported that alginate-catechol (Al-CA) hydrogel can provide high mechanical strength and chemical stability without deformation over a wide range of pH values [8]. There is no up-to-date study examining the suitability of Al-CA hydrogel in encapsulating islet cells; in this short communication, we report whether Al-CA is suitable material for islet transplantation.
Twelve-week-old C57BL/6 mice (Envigo, Horst, the Netherlands) maintained under specific pathogen free conditions were euthanized, and pancreatic islet isolation was performed as previously introduced [9]. All animal care and all experimental procedures were approved by the Institutional Animal Care and Use Committee Health System (IACUC, #2015-0164) of Yonsei University College of Medicine.
The Al-CA conjugate was synthesized by chemical reaction using 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDC) and N-hydroxysulfosuccinimide (NHS). To conjugate the dopamine, alginate (FMC Biopolymer, Philadelphia, PA, USA) was dissolved in distilled water at a concentration of 1% (w/v). EDC (Thermo Scientific, Waltham, MA, USA) and NHS (Sigma, St. Louis, MO, USA) were then added to the 1% alginate solution. Dopamine hydrochloride (Sigma) was added to the solution at a 1:1 molar ratio of dopamine hydrochloride to alginate at pH 5. The reaction was performed at room temperature overnight. The reaction mixture was dialyzed using Dulbecco's phosphate buffered saline (DPBS) and acidic distilled water (pH 5) and was then freeze-dried. To produce Al-CA hydrogels, the catechol moieties conjugated to the alginate backbone were cross-linked with an oxidizing agent. Al-CA dissolved in DPBS (at 4%) was mixed with a gelation solution containing 0.4 M sodium hydroxide and an oxidizing agent (sodium periodate: 4.5 mg/mL, equal molar ratio to catechol) to initiate cross-linking. Alginate without the catechol modification was cross-linked by adding 100 mM calcium chloride (CaCl2). For a negative control, we used photo-cross-linked hyaluronic acid hydrogel by methacrylate and collagen as previously introduced [10].
To examine cytotoxicity, 15 isolated islet cells encapsulated by Al-CA hydrogel were stained with acridine orange (AO) and propidium iodide (PI) in DPBS for 30 minutes at 37.0℃ with 5% CO2 on the day of isolation, and on the 3rd, 5th, and 7th days following isolation; then, the cells were observed by confocal microscopy. Thirty encapsulated islets with Al-CA hydrogel underwent glucose-stimulated insulin secretion (GSIS), as described elsewhere [9]. The islets were pre-incubated in Krebs-Ringer buffered solution without glucose for 30 minutes, and, then they were stimulated by the same buffer containing 30 mM glucose for 30, 45, and 60 minutes. The supernatants were examined for insulin by enzyme-linked immunosorbent assay (ELISA; ALPCO, Salem, NH, USA).
Gelation of alginate occurs when its carboxyl groups are stably crossed-linked with multivalent cations, such as Ca2+ [711]. In addition, by considering that cross-linking with Ca2+ provides the hydrogel high biostability [12], we used Al-Ca2+ hydrogel as a reference encapsulating material. Al-CA hydrogel successfully encapsulated islet cells for 7 days without leakage or collapse (Fig. 1A), and, as shown in Fig. 1B, the gel itself merely affected the viability of islet cells, as evidenced by no PI signal (red) and intact AO signal (green) in the islet cells over the 7 observation days.
Both under 0 mM glucose (P<0.001) and high glucose (30 mM) stimulating conditions (P<0.001), Al-CA hydrogel negatively affected insulin secretion (Fig. 2A) compared with free islet cells. Although 30 mM glucose induced insulin response in the encapsulated islet cells (P<0.001), the secreted insulin level was even lower than that of non-stimulated free islet cells. Insulin responses in the encapsulated islets were also significantly attenuated during long-term exposure to hyperglycemic conditions compared with those of non-stimulated free islet cells (Fig. 2B).
In this study, we demonstrated that encapsulation of murine pancreatic islet cells with Al-CA hydrogel does not induce cytotoxicity ex vivo for an extended period; however, it markedly abated GSIS.
Alginate hydrogels are used in various biomedical applications such as tissue engineering, cell therapy, and drug delivery. For islet encapsulation, alginate is recently considered to be one of the most suitable biomaterials owing to its availability, cost, ease of production, and, more importantly, its properties of not hampering hormonal secretion from islets and increasing islet survival in rodent models [613]. However, control for swelling or viscoelastic and biophysical properties of alginate hydrogels prepared by conventional cross-linking methods with multivalent cations or other synthetic polymers often led to decreased biocompatibility [14]. To overcome these problems, a gelation process of cross-linking alginate with catechol was introduced; in fact, Al-CA hydrogels appear to enhance survival times of various human primary cells with imperceptible intrinsic cytotoxicity [14]. This may be due to the biophysical and chemical properties, which yield high mechanical strength and chemical stability over a wide range of pH values [8]. We also observed marginal cytotoxicity and prolonged ex vivo survival of encapsulated islet cells with Al-CA hydrogels; however, the hydrogels greatly interfered with the insulin response. A previous study using chitosan polyethylene for cross-linking to alginate for gelation reported that islet cells encapsulated with Ca2+-alginate hydrogel secreted more insulin than the other two in response to glucose, although the stability in acidic condition was the lowest [15]. These results suggested that cross-linking constituents for alginate gelation can significantly negatively influence the insulin secretion in islet cells, although they can enhance biochemical and/or biophysical stability. A major requirement for clinical application of encapsulated islet cells, in addition to the mechanical stability of the capsules to stay intact and to protect from the host's immune system, is the diffusion kinetics for glucose; in fact, a delay in GSIS has been observed early in vitro and in vivo studies [1617]. A possible explanation for the impaired insulin secretion by Al-CA cross-linking in our study is that Al-CA hydrogel may interfere with membrane proteins of islet cells, such as glucose transporters, and ion channels, which are important in insulin secretory machinery. A recent study reported that the increased adhesion property of the hydrogel by catechol can induce strong attachments between the gel surface and the cell membrane [18]. Considering our results, cellular permeability of alginate for glucose and insulin may be decreased by this strong attachment; therefore, catechol should not be considered as a constituent for alginate gelation for encapsulating islet cells in the application of islet transplantation. Further investigations for the identification of the cross-linking constituent for alginate gelation are necessary.
Figures and Tables
Fig. 1
Cytotoxicity test for encapsulated islet cells. Isolated murine islet cells were encapsulated with alginate hydrogel cross-linked either by calcium ion (Al-Ca2+) or catechol (alginate-catechol [Al-CA]). (A) Microscopic image of encapsulated islet cells with Al-CA hydrogel on the 7th day after the encapsulation. (B) Acridine orange and propidium iodide (PI) staining at different days post-encapsulation. No PI signal over 7 days post-encapsulation was detected. HA-ME+Col, hyaluronic methacrylate+collagen.
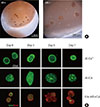
Fig. 2
Glucose-stimulated insulin secretion from encapsulated islet cells. Isolated murine islet cells were encapsulated with alginate hydrogel cross-linked by catechol (alginate-catechol [Al-CA]). (A) Free islet cells or encapsulated islet cells with Al-CA hydrogel (Al-CA encapsulated islets) were stimulated by 30 mM glucose for 30 minutes after 30-minute pre-incubation with 0 mM glucose. (B) Longer exposure to hyperglycemic milieu (30 mM) in both conditioned islet cells. aSignificantly higher than 0 mM glucose stimulation (P<0.001), bSignificantly lower than corresponding conditions in free islets (P<0.001), cSignificantly lower than corresponding conditions in free islets (P<0.001).

ACKNOWLEDGMENTS
This study was funded by faculty research grant of Yonsei University College of Medicine (6-2009-0065).
References
1. Maahs DM, West NA, Lawrence JM, Mayer-Davis EJ. Epidemiology of type 1 diabetes. Endocrinol Metab Clin North Am. 2010; 39:481–497.

2. You WP, Henneberg M. Type 1 diabetes prevalence increasing globally and regionally: the role of natural selection and life expectancy at birth. BMJ Open Diabetes Res Care. 2016; 4:e000161.

3. Shapiro AM, Pokrywczynska M, Ricordi C. Clinical pancreatic islet transplantation. Nat Rev Endocrinol. 2017; 13:268–277.

4. Shapiro AM. State of the art of clinical islet transplantation and novel protocols of immunosuppression. Curr Diab Rep. 2011; 11:345–354.

5. Desai T, Shea LD. Advances in islet encapsulation technologies. Nat Rev Drug Discov. 2017; 16:367.

6. Souza YE, Chaib E, Lacerda PG, Crescenzi A, Bernal-Filho A, D'Albuquerque LA. Islet transplantation in rodents. Do encapsulated islets really work? Arq Gastroenterol. 2011; 48:146–152.

7. de Vos P, Hamel AF, Tatarkiewicz K. Considerations for successful transplantation of encapsulated pancreatic islets. Diabetologia. 2002; 45:159–173.

8. Lee YK, Lee SY. A colorimetric alginate-catechol hydrogel suitable as a spreadable pH indicator. Dyes Pigm. 2014; 108:1–6.

9. Carter JD, Dula SB, Corbin KL, Wu R, Nunemaker CS. A practical guide to rodent islet isolation and assessment. Biol Proced Online. 2009; 11:3–31.

10. Baier Leach J, Bivens KA, Patrick CW Jr, Schmidt CE. Photocrosslinked hyaluronic acid hydrogels: natural, biodegradable tissue engineering scaffolds. Biotechnol Bioeng. 2003; 82:578–589.

11. Sakata N, Sumi S, Yoshimatsu G, Goto M, Egawa S, Unno M. Encapsulated islets transplantation: past, present and future. World J Gastrointest Pathophysiol. 2012; 3:19–26.

12. Cui J, Wang M, Zheng Y, Rodriguez Muniz GM, del Campo A. Light-triggered cross-linking of alginates with caged Ca2+. Biomacromolecules. 2013; 14:1251–1256.

13. Giri TK, Thakur D, Alexander A, Ajazuddin , Badwaik H, Tripathi DK. Alginate based hydrogel as a potential biopolymeric carrier for drug delivery and cell delivery systems: present status and applications. Curr Drug Deliv. 2012; 9:539–555.
14. Lee C, Shin J, Lee JS, Byun E, Ryu JH, Um SH, Kim DI, Lee H, Cho SW. Bioinspired, calcium-free alginate hydrogels with tunable physical and mechanical properties and improved biocompatibility. Biomacromolecules. 2013; 14:2004–2013.

15. Roshanbinfar K, Salahshour Kordestani S. Encapsulating beta islet cells in alginate, alginate-chitosan and alginate-chitosan-PEG microcapsules and investigation of insulin secretion. J Biomater Tissue Eng. 2013; 3:185–189.

16. Korsgren O. Islet encapsulation: physiological possibilities and limitations. Diabetes. 2017; 66:1748–1754.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download