Abstract
Background
Methods
Results
References
Fig. 1
Study flow chart. AMI, acute myocardial infarction; PCI, percutaneous coronary intervention; T2DM, type 2 diabetes mellitus; T1DM, type 1 diabetes mellitus; MI, myocardial infarction.
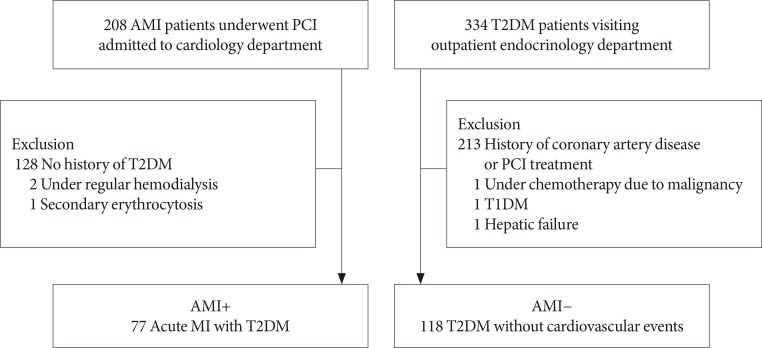
Fig. 2
Schema of the measurement of the red blood cell. Elongation index (EI), which was measured at a pressure of 3 Pa, was defined as (L−W)/(L+W) and it was expressed as a percentage. L means the length of the major axis of the cell, and W means the width of the minor axis of the cell. The lower the value of the EI is, the more circular the cell becomes.
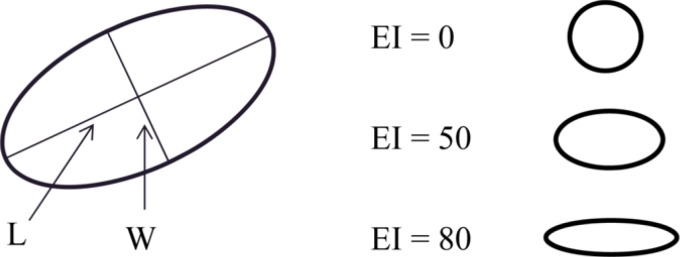
Fig. 3
Comparison of erythrocyte functions (A, elongation index, P<0.001; B, critical shear stress, P=0.040) between two groups. AMI, acute myocardial infarction. aAMI group showed lower elongation index (A, 30.44%±1.77% vs. 31.47%±1.48%) and higher critical shear stress (B, 316.13±108.20 mPa vs. 286.80±85.34 mPa) compared to control group.
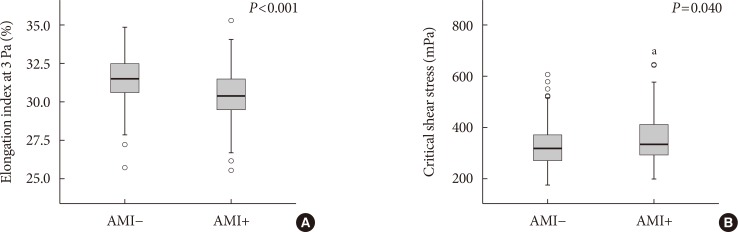
Fig. 4
Correlation between the renal function and elongation index at 3 Pa (A, r=0.375, P<0.001; black square, acute myocardial infarction [AMI] group; empty square, non-AMI group) and critical shear stress (B, r=−0.317, P<0.001). GFR, glomerular filtration rate.
Fig. 5
Correlation between the elongation index at 3 Pa and critical shear stress (r=−0.124, P=0.090; black square, acute myocardial infarction [AMI] group; empty square, non-AMI group).
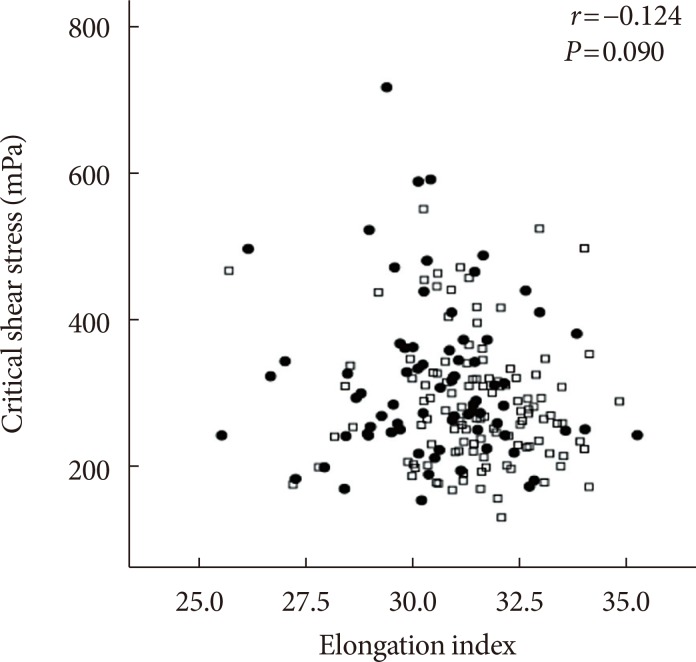
Fig. 6
Correlation between glycosylated hemoglobin and the elongation index at 3 Pa (r=−0.161, P=0.029; black square, acute myocardial infarction [AMI] group; empty square, non-AMI group).
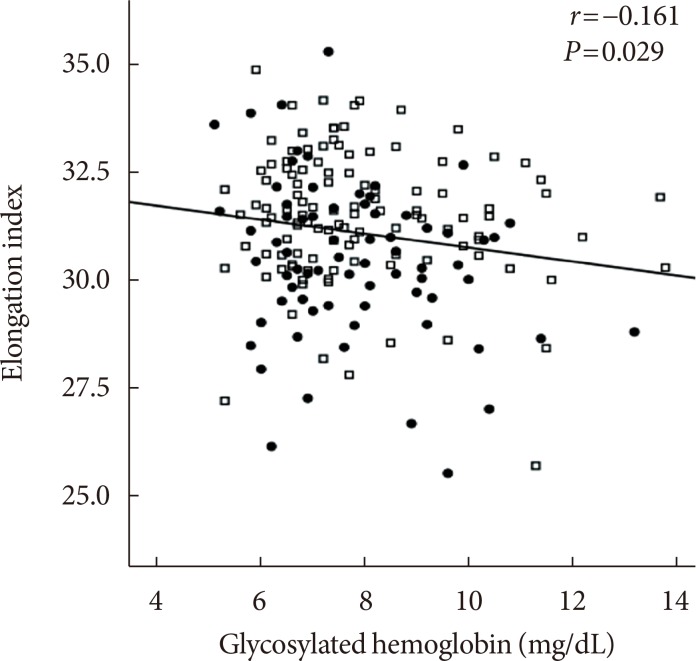
Table 1
Baseline characteristics
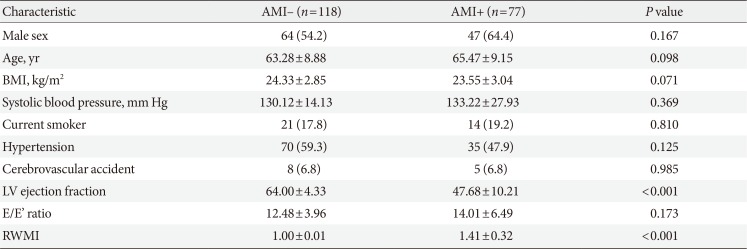
Table 2
Laboratory findings
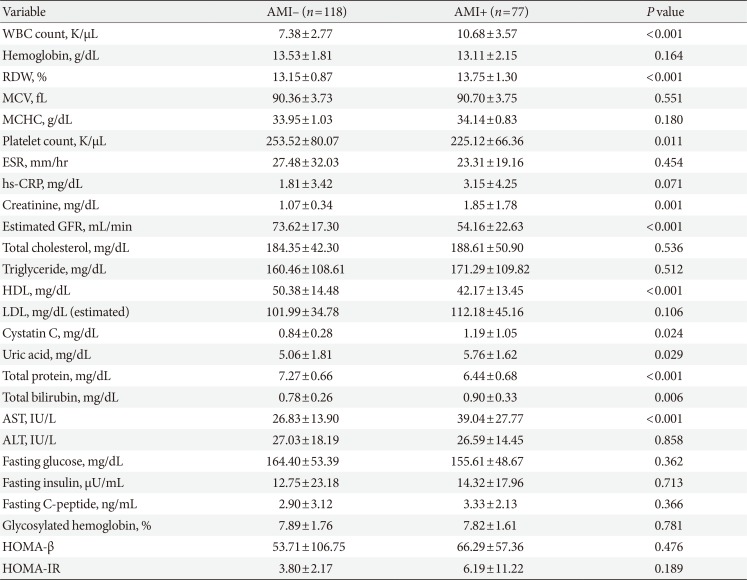
Values are presented as mean±standard deviation.
AMI, acute myocardial infarction; WBC, white blood cell; RDW, red cell distribution width; MCV, mean corpuscular volume; MCHC, mean corpuscular hemoglobin concentration; ESR, erythrocyte sedimentation rate; hs-CRP, high-sensitivity C-reactive protein; GFR, glomerular filtration rate; HDL, high density lipoprotein; LDL, low density lipoprotein; AST, aspartate aminotransferase; ALT, alanine aminotransferase; HOMA-β, homeostatic model assessment of β-cell; HOMA-IR, homeostatic model assessment of insulin resistance.
Table 3
The association between critical shear stress and erythrocyte sedimentation rate or dipeptidyl peptidase-4 inhibitors on multiple linear regression

| Variable | R2 | Estimated β | Standard error | P value |
|---|---|---|---|---|
| Erythrocyte sedimentation rate | 0.497 | 3.644 | 0.734 | <0.001 |
| Dipeptidyl peptidase-4 inhibitors | 0.574 | −64.215 | 30.872 | 0.048 |
Adjusted for sex, age, hypertension, history of cerebrovascular accident, smoking, body mass index, systolic blood pressure, pharmacologic status (nicorandil, insulin, metformin, aspirin, adenosine diphosphate receptor blocker, renin-angiotensin system blockade, β-blocker, calcium channel blocker, 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitor, sulfonylurea, dipeptidyl peptidase-4 inhibitors), left ventricular ejection fraction, E/E′ ratio, white blood cell, hemoglobin, red cell distribution width, mean corpuscular volume, mean corpuscular hemoglobin concentration, platelet count, high-sensitivity C-reactive protein, total protein, aspartate transaminase, alanine transaminase, estimated glomerular filtration rate, glycosylated hemoglobin, total cholesterol, cystatin C, homeostasis model assessment β-cell, and homeostasis model assessment insulin resistance.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download