Abstract
Background
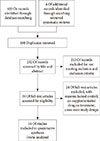
Methods
Results
Figures and Tables
Fig. 2
Risk of bias assessment tool. (A) Summary of risk of bias of across the trials included in the systematic review. Information for every study characteristic was pooled from every trial (green: low risk of bias; yellow: unclear risk of bias; red: high risk of bias), combined and overall results expressed in percentages. (B) Overview of risk of bias across individual trials according to study characteristics. Each bias domain was evaluated carefully from every trial and decided whether the information provided reflected a low risk of bias (green), high risk of bias (red), or if insufficient information was provided and the risk of bias was therefore unclear (yellow).

Fig. 3
Influence of omega-3 fatty acids supplementation on plasminogen-activator inhibitor 1 levels (ng/mL). Forest plot shows pooled mean differences with 95% confidence intervals (CIs) for two randomized controlled trials. The green colored square represents the point estimate of the effect of the intervention for each trial. The horizontal line joins the upper and lower limits of the 95% CI of the effects. The square area represents the relative weight of the trial in the meta-analysis. The black colored diamond at the bottom represents the pooled mean difference with 95% CI for all study groups. SD, standard deviation; IV, interval variable.

Fig. 4
Influence of omega-3 fatty acids supplementation on adiponectin levels (µg/mL). (A) Forest plot shows pooled mean differences with 95% confidence intervals (CIs) for 10 randomized controlled trials. The green colored square represents the point estimate of the effect of the intervention for each trial. The horizontal line joins the upper and lower limits of the 95% CI of the effects. The square area represents the relative weight of the trial in the meta-analysis. The black colored diamond at the bottom represents the pooled mean difference with 95% CI for all study groups. As opposed to graphs for all other outcome parameters, the labels of the X-axis are different, because an increase in adiponectin levels is seen as favorable. (B) Meta-analysis after eliminating Veleba et al. [43] as part of the leave-one-out sensitivity analysis (the trial was given a relative weight of 0.0%). SD, standard deviation; IV, interval variable.

Fig. 5
Influence of omega-3 fatty acids supplementation on resisting levels (ng/mL). Forest plot shows pooled mean differences with 95% confidence intervals (CIs) for two randomized controlled trials. The green colored square represents the point estimate of the effect of the intervention for each trial. The horizontal line joins the upper and lower limits of the 95% CI of the effects. The square area represents the relative weight of the trial in the meta-analysis. The black colored diamond at the bottom represents the pooled mean difference with 95% CI for all study groups. SD, standard deviation; IV, interval variable.

Fig. 6
Influence of omega-3 fatty acids supplementation on leptin levels (ng/mL). (A) Forest plot shows pooled mean differences with 95% confidence intervals (CIs) for seven randomized controlled trials. The green colored square represents the point estimate of the effect of the intervention for each trial. The horizontal line joins the upper and lower limits of the 95% CI of the effects. The square area represents the relative weight of the trial in the meta-analysis. Notice the absence of the black colored diamond at the bottom as in other graphs because of the magnitude of the pooled mean difference with 95% CI for all study groups. (B) Meta-analysis after eliminating Spencer et al. [41] as part of the leave-one-out sensitivity analysis (the trial was given a relative weight of 0.0%). SD, standard deviation; IV, interval variable.

Fig. 7
Influence of omega-3 fatty acids supplementation on interleukin 6 levels (pg/mL). Forest plot shows pooled mean differences with 95% confidence intervals (CIs) for eight intervention effects pooled from seven randomized controlled trials (two separate effects were pooled for docosahexaenoic acid [DHA] and eicosapentaenoic acid [EPA] treatment arms from Mori et al. [38]). The green colored square represents the point estimate of the effect of the intervention for each intervention. The horizontal line joins the upper and lower limits of the 95% CI of the effects. The square area represents the relative weight of the interventions in the meta-analysis. SD, standard deviation; IV, interval variable.

Fig. 8
Influence of omega-3 fatty acids supplementation on tumor necrosis factor α levels (pg/mL). (A) Forest plot shows pooled mean differences with 95% confidence intervals (CIs) for nine intervention effects pooled from eight randomized controlled trials (two separate effects were pooled for docosahexaenoic acid [DHA] and eicosapentaenoic acid [EPA] treatment arms from Mori et al. [38]). The green colored square represents the point estimate of the effect of the intervention for each intervention. The horizontal line joins the upper and lower limits of the 95% CI of the effects. The square area represents the relative weight of the interventions in the meta-analysis. (B) Meta-analysis after eliminating Spencer et al. [41] as part of the leave-one-out sensitivity analysis (the trial was given a relative weight of 0.0%). SD, standard deviation; IV, interval variable.

Fig. 9
Subgroup analysis on the influence of omega-3 fatty acids supplementation according to (A) omega-3 dose and (B) treatment duration for adiponectin (µg/mL). Forest plot shows pooled mean differences with 95% confidence intervals (CIs). The green colored square represents the point estimate of the effect of the intervention for each trial. The horizontal line joins the upper and lower limits of the 95% CI of the effects. The square area represents the relative weight of the trial in the meta-analysis. The black colored diamond at the bottom represents the pooled mean difference with 95% CI for all study groups. SD, standard deviation; IV, interval variable.

Fig. 10
Subgroup analysis on the influence of omega-3 fatty acids supplementation according to (A) omega-3 dose and (B) treatment duration for tumor necrosis factor α (pg/mL). Forest plot shows pooled mean differences with 95% confidence intervals (CIs). The green colored square represents the point estimate of the effect of the intervention for each trial. The horizontal line joins the upper and lower limits of the 95% CI of the effects. The square area represents the relative weight of the trial in the meta-analysis. The black colored diamond at the bottom represents the pooled mean difference with 95% CI for all study groups. SD, standard deviation; IV, interval variable; DHA, docosahexaenoic acid; EPA, eicosapentaenoic acid.

Fig. 11
Funnel plot displaying study precision against the mean difference (MD) effect estimate with 95% confidence interval for (A) adiponectin, (B) leptin, (C) interleukin 6, and (D) tumor necrosis factor α. SE, standard error.

Table 1
General characteristics of randomized controlled trials included in the systematic review

| Reference | Sample size, % female subjects | Age, yr | BMI, kg/m2 | Duration, design | Disease, medication | Intervention (daily EPA/DHA dose) | Control group | Outcome, parameters |
|---|---|---|---|---|---|---|---|---|
| Hashemi et al. (2014) [30] | 70 (I: 41, C: 36) | I: 44±5.0 | I: 27.9±1.7 | 12 Weeks, parallel | T2DM subjects; treated with biguanides and sulfonylureas (76%), sulfonylureas (16%) or biguanides alone (8%) | EPA pearls (2,000 mg) | Placebo (corn oil) | Adiponectin |
| C: 45±4.1 | C: 27.8±1.6 | |||||||
| Jacobo-Cejudo et al. (2017) [31] | 54 (I: 70.6, C: 83.9) | I: 50.4±6.3 | I: 25.6±2.4 | 24 Weeks, parallel | T2DM; therapy with glibenclamide+metformin or metformin alone, received no additional dietary or lifestyle advice | Fish oil (320 mg EPA, 200 mg DHA) | Placebo (cornstarch) | Adiponectin, leptin, resistin |
| C: 48.1±6.9 | C: 26.0±1.6 | |||||||
| Kabir et al. (2007) [32] | 26 (all female) | I: 55±2.0 | 30±2.0 in both groups | 2 Months, parallel | Postmenopausal T2DM women without hypertriglyceridemia; 3 patients treated only with diet, 23 taking oral hypoglycemic treatment (7 only biguanides, 16 sulfonylureas and biguanides), 5 lipid-lowering agents, 6 hormone replacement therapy | Fish oil (1,080 mg EPA, 720 mg DHA) | Placebo (paraffin oil) | Adiponectin, leptin, TNF-α, IL-6, PAI-1 |
| C: 55±1.0 | ||||||||
| Krebs et al. (2006) [33] | 67 (all female) | 44.7±13.2 | 35.0±5.5 | 24 Weeks, parallel | Overweight or obese women with hyperinsulinemia | Fish oil (1,300 mg EPA, 2,900 mg DHA)+weight loss intervention (10% of body weight loss in 12 weeks, 12 weeks weight maintenance) | 5 g oil/day totaling 2.8 g linoleic acid and 1.4 g oleic acid+weight loss intervention (10% of body weight loss in 12 weeks, 12 weeks weight maintenance) | Adiponectin, leptin, TNF-α, IL-6 |
| Lee et al. (2014) [34] | 37 (I: 62.5, C: 71.4) | I: 56.2±8.7 | I: 33.2±4.8 | 8 Weeks, parallel | Early stage T2DM (no evidence of end-stage organ damage secondary to diabetes) | Fish oil (3,580 mg EPA, 2,440 mg DHA) | Placebo (corn oil) | Leptin |
| C: 59.9±9.8 | C: 34.8±5.3 | |||||||
| Mazaherioun et al. (2017) [35] | 85 (I: 34.09, C: 41.4) | I: 51.5±7.45 | I: 29.22±3.58 | 10 Weeks, parallel | T2DM; no insulin, thiazolidinediones or anti-obesity medications | Omega-3 capsules (1,800 mg EPA, 900 mg DHA) | Placebo (paraffin) | Adiponectin |
| C: 50.56±7.21 | C: 29.21±2.90 | |||||||
| Mocking et al. (2012) [36] | 24 (I: 62, C: 42) | I: 53.1±13.8 | I: 29.3±5.1 | 12 Weeks, parallel | Diabetic patients with major depressive disorder (determined by the Composite International Diagnostic Interview standards); therapy with antidepressants for at least 2 months, diabetes treated with diet only, hypoglycemics, insulin, or both | Fish oil (at least 900 mg ethyl-EPA) | Placebo (rapeseed oil+medium chain triglycerides) | TNF-α, IL-6 |
| C: 55.0±8.6 | C: 29.8±4.8 | |||||||
| Malekshahi Moghadam et al. (2012) [37] | 84 (50% in both groups) | I: 55.36±9.88 | I: 27.72±3.47 | 8 Weeks, parallel | T2DM subjects (at least 2 years since diagnosis), all receiving anti-hyperglycemic treatment | Omega-3 capsules (1,548 mg EPA, 828 mg DHA) | Placebo (sunflower oil) | TNF-α |
| C: 52.96±10.72 | C: 27.51±3.16 | |||||||
| Mori et al. (2003) [38]a | 51 (I1: 17,6, I2: 27,7, C: 25) | I1: 61.2±2.3 | I1: 30.6±0.7 | 6 Weeks, parallel | Non-smoking, treated-hypertensive, T2DM men and postmenopausal women; antihypertensive therapy for a minimum of 3 months, oral hypoglycemic, but no insulin therapy | I1: 4 g EPA-capsules per day (96% EPA ethyl ester) | Placebo (olive oil) | TNF-α, IL-6 |
| I2: 60.9±1.9 | I2: 27.9±0.8 | I2: 4 g DHA per day (92% DHA ethyl ester) | ||||||
| C: 61.5±1.9 | C: 29.9±1.0 | |||||||
| Ogawa et al. (2013) [39] | 26 (I: 69, C: 82) | I: 79.5±8.6 | I: 19.9±4.0 | 3 Mllel | Bedridden elderly T2DM patients | Liquid diet rich in EPA (25 mg/100 kcal) and DHA (17 mg/100 kcal)b | Liquid diet lacking EPA and DHA | Adiponectin, TNF-α, IL-6 |
| C: 81.2±7.6 | C: 20.1±3.6 | |||||||
| Poreba et al. (2017) [40] | 74 (I: 38,9, C: 31.6) | I: 64.4±6.7 | I: 30.9 (27.9–34.7) | 3 Months, parallel | T2DM patients with a history of coronary artery disease or peripheral artery disease | Omega-3 drink (1,000 mg EPA, 1,000 mg DHA) | Drink without omega-3 | Adiponectin, leptin, TNF-α, IL-6 |
| C: 66.7±6.8 | C: 31.1 (28.1–32.7)c | |||||||
| Spencer et al. (2013) [41] | 33 (I: 68,4, C: 64,2) | I: 48.8±2.3 | I: 33.4±2.3 | 12 Weeks, parallel | Non-diabetic subjects with either impaired fasting glucose, impaired glucose tolerance (23 subjects), or at least three signs of metabolic syndrome | Fish oil (1,860 mg EPA, 1,500 mg DHA) | Placebo (corn oil) | Adiponectin, leptin, TNF-α, IL-6, PAI-1, resistin |
| C: 53.3±2.2 | C: 33.4±1.1 | |||||||
| Stirban et al. (2014) [42] | 34, No specification of sex | 56.8±8.3 | 31.2±4.1 | 6 Weeks, cross-over | T2DM (duration 9.8± 6.6 years), no history of major cardiovascular events or a surgical or interventional history within the previous 6 months; treated with low dose aspirin (n=20), ACE-inhibitors or AR blockers (n=24), calcium channel blockers (n=5), β-blockers (n=15), diuretics (n=13), and statins (n=13), all withdrawn before measurements; diabetes medication: diet (n=3), oral therapy (n=16), insulin (n=4), oral hypoglycemic plus insulin (n=10), and incretin mimetics (n=1) | Omega-3 capsules (920 mg EPA, 760 mg DHA) | Placebo (olive oil) | Adiponectin, leptin |
| Veleba et al. (2015) [43] | 29, 34 | I: 59.5 | I: 34.0 | 24 Weeks, parallel | T2DM (at least 3 months from diagnosis); metformin as monotherapy as a stable dose (0.5–3.0 g/day) for at least 1 month | Omega-3 concentrate (750 mg EPA, 2,000 mg DHA) | Placebo (corn oil) | Adiponectin, leptin |
| C: 62.0 | C: 30.9 | |||||||
| Wong et al. (2013) [44] | 25, 44 | 60±4.0 | I: 34±2.0 | 16 Weeks, parallel | Middle-aged, centrally obese, normotensive, dyslipidemic (elevated triglycerides, low HDL), insulin resistant adults; 6 subjects receiving lipid-lowering therapy (4 atorvastatin, 2 rosuvastatin), 5 anti-hypertensive treatment (2 on ACE inhibitors, 2 on AR blockers, 1 on β1-blocker) | Fish oil (1,840 mg EPA-ethyl ester, 1,520 mg ethyl-ester)+weight loss intervention (12 weeks hypocaloric diet with energy deficit of at least 1,900 kJ/day, followed by 4 weeks of weight maintenance with energy intake increased by 460 kJ/day) | Weight loss alone (protocol as in intervention group) | Adiponectin |
| C: 33±1.0 |
Values are presented as mean±standard deviation.
BMI, body mass index; I, intervention group; C, control group; T2DM, type 2 diabetic mellitus; EPA, eicosapentaenoic acid; DHA, docosahexaenoic acid; TNF-α, tumor necrosis factor α; IL-6, interleuk in 6; PAI-1, plasminogen-activator inhibitor 1; ACE, angiotensin-converting-enzyme; AR, angiotensin II receptor; HDL, high density lipoprotein.
aEPA and DHA were used in two separate treatment arms, bThe patients consumed 1,015.4±231.1 kcal/day, cValues are presented as median (interquartile range).




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download