Abstract
Transient receptor potential canonical 4 (TRPC4) channel is a nonselective calcium-permeable cation channels. In intestinal smooth muscle cells, TRPC4 currents contribute more than 80% to muscarinic cationic current (mIcat). With its inward-rectifying current-voltage relationship and high calcium permeability, TRPC4 channels permit calcium influx once the channel is opened by muscarinic receptor stimulation. Polyamines are known to inhibit nonselective cation channels that mediate the generation of mIcat. Moreover, it is reported that TRPC4 channels are blocked by the intracellular spermine through electrostatic interaction with glutamate residues (E728, E729). Here, we investigated the correlation between the magnitude of channel inactivation by spermine and the magnitude of channel conductance. We also found additional spermine binding sites in TRPC4. We evaluated channel activity with electrophysiological recordings and revalidated structural significance based on Cryo-EM structure, which was resolved recently. We found that there is no correlation between magnitude of inhibitory action of spermine and magnitude of maximum current of the channel. In intracellular region, TRPC4 attracts spermine at channel periphery by reducing access resistance, and acidic residues contribute to blocking action of intracellular spermine; channel periphery, E649; cytosolic space, D629, D649, and E687.
Transient receptor potential canonical (TRPC) channel is a Ca2+-permeable, nonselective cation channel in a mammalian cell and involved in lots of physiological functions such as endothelial permeability, salivary gland secretion, gastrointestinal (GI) motility and many others [12]. The TRPC channel family was composed of seven types, i.e., TRPC1 to TRPC7 and subgroup could be classified based on their amino acid sequence homology where TRPC1, 4, and 5 were classified as one subgroup while TRPC3, 6, and 7 were classified as the other. One of the most prominent physiological functions was the role of TRPC4 and TRPC6 in gastrointestinal physiology, especially in ileum myocytes [345].
Muscarinic cationic current (mIcat), a cationic current evoked by muscarinic stimulation, had been deeply analyzed to identify its molecular candidates. Recent studies presented conclusive evidence that TRPC4 and TRPC6 function as two separate channels responsible for mIcat [5]. Their findings suggest that TRPC4 and TRPC6 channels are functionally coupled with muscarinic receptors and provokes depolarization of intestinal smooth muscle once the receptors are activated by cholinergic stimulation. Tsvilovskyy et al. [5] showed that, in intestinal smooth muscle cells, TRPC4 currents contribute more than 80% to mIcat and TRPC6 contribute the remaining proportion. In the single TRPC knockouts, there also seemed to be no overlap of or compensation between TRPC4 and TRPC6 currents whereas mIcat was completely eliminated in the TRPC4/TRPC6 double knockouts cells.
Due to low selectivity among monovalent cations [6], TRPC4 channel show reversal potential near 0 mV [78]. Moreover, the I–V relationship of the channel clearly shows that the channel permits strong inward cationic current as long as the channel is properly opened by appropriate activators such as functionally coupled GPCR activation [6], overexpression of gain-of-function mutant of heterotrimeric G-proteins, intracellular dialysis of GTPγS [9], extracellular application of selective TRPC activator (−)-Englerin A [10], and many others. In addition to strong inward current, TRPC4 channels show very low cationic conductance from 0 mV up to 40 mV [7]. Considering physiological membrane potential range, it would be reasonable to view TRPC4 channel as a strong inward rectifier and mild membrane depolarizer [7]. Such biophysical properties of TRPC4 channels are similar to inwardly-rectifying potassium channels (IRK) except for high potassium selectivity and corresponding hyperpolarizing Nernst potential of IRK. Stark difference between two channels is that there is absolutely no current at highly positive voltage in IRK but TRPC4 channels show outward burst of cationic current at highly positive voltages (higher that 50mV). Studies for mechanism of inward rectification of IRK has been done intensively, and two cytoplasmic blocking agents, Mg2+ and polyamines, have been considered as key molecules for such rectification. In essence, both cationic molecules plug the cytoplasmic vestibule [111213], or even central ion conducting pathway of IRK [14], and prohibits outward flux of potassium ions. In addition, there have been debates how the key molecules and their binding to the channel are correlated to distinct biophysical properties of blocking such as voltage dependency (k, V1/2), dissociation constant (Kd), electrical distance of blocking agent (zδ), and many others [15]. Atomic structures of inwardly-rectifying potassium channels now clearly show structure-function relationship of voltage-dependent blocking action of spermine [14], however, earlier studies had postulated similar thesis long before structural insight was given. In particular, studies showed that increasing extracellular potassium concentration decreases the magnitude of inward rectification [1617]. It was further elaborated that such phenomenon was occurred since at depolarized potentials, spermine moves up toward outer borderline of plasma membrane and essentially plugs central ion conducting pathway. Authors of the study explained that increased extracellular potassium concentration makes the channel pore crowded with potassium ions, leaving positively charged blocking agents knocked down in competition for negatively charged residues. The insight given here was further extended to explaining increased size of ‘hump’ in IRK at hyperkalemic condition.
In late 1990s and early 2000s, early studies questioning inward-rectification mechanism of TRPC channels or effect of polyamines to near-equivalent mIcat have been started [1819]. It was reported that concentration of spermine at extracellular fluid of ileal myocyte is about 0.3 mM and such extracellular spermine reduces mIcat. In TRPC5 channels, possible cytoplasmic Mg2+ binding sites were revealed: D633 and D636. Site-directed mutagenesis of those residues to alanine or asparagine abolished inward rectification of TRPC5 channels and I–V curve of mutant channels showed ohmic relationship.
Recently, we have shown that intracellular spermine strongly blocks TRPC4 channels and inhibits outward cationic current [20]. We also revealed that molecular nature of such inhibition was electrostatic interaction between positive charge of amines (−NH3+) and cytoplasmic negative charge of carboxylic acids of glutamates (E728, E729, −COO−). Although we have found that spermine-mediated inhibition of outward current was reduced when E728, E729 were mutated to alanine, the mutations could not fully recover outward current from spermine.
Here, we studied if there exist additional residues in TRPC4 crucial for interaction with intracellular spermine. We first started with aspartate at 629th residue (D629) which is the homologous site for D633 in TRPC5. We also studied whether increased conductance affects degree of inhibition by spermine, which may give the clue if the interaction site is inside the central ion conducting pathway. Last but not the least, we investigated whether putative spermine-binding sites found by electrophysiology have structural significance based on recently published Cryo-EM data of mouse TRPC4 [21].
Human embryonic kidney (HEK293) cells (ATCC, Manassas, VA, USA) were maintained according to the supplier's recommendations. HEK293 cells were cultured for 3 or 4 days depending on their confluency and passages from 5 to 15 were used. For transient transfection, cells were seeded in 12-well plates. The following day, 0.5–2.5 µg/well of pcDNA vector containing the cDNA for mTRPC4β-EGFP was transfected into the cells using the transfection reagent FuGENE 6 (Roche Molecular Biochemicals, Indianapolis, IN, USA) according to the manufacturer's protocol. After 24–30 h, the cells were trypsinized with Trypsin EDTA and used for whole-cell recording.
Plasmid containing mouse TRPC4β gene was kindly donated by Dr. M. Schaefer in Leipzig, Germany. Site-directed mutations in TRPC4 were introduced using the QuickChange site-directed mutagenesis kit (Stratagene, La Jolla, CA, USA) and appropriate primer sets. Sequences of the mutants were confirmed by DNA sequencing.
The cells were transferred to a small chamber on the stage of an inverted microscope (Eclipse Ti; Nikon, Tokyo, Japan) attached for 10 min before recording. Glass microelectrodes with 2–2.5 MΩ resistance were used to obtain giga-ohm seals. The bath solutions were constantly perfused with an extracellular solution at a rate of 1–2 ml/min. The currents were recorded using an Axopatch 200B patch clamp amplifier (Axon Instruments, San Jose, CA, USA). The current was recorded for 500-ms duration ramps from +100 to 100 mV with a time resolution of 0.4 mV/ms and with a holding membrane potential of −60 mV). For recordings of the TRPC channels, we used normal Tyrode solution (NT) unless otherwise mentioned and occasionally Cs+-rich external solution. pCLAMP software v.10.2 and a Digidata 1440A (Axon Instruments) were used for data acquisition and application of the command pulses. The data were filtered at 2 kHz and displayed on a computer monitor.
Structural analysis was based on the Cryo-EM structure of mouse TRPC4 [21]. The modeled 3-D structures of TRPC4 mutants were visualized and analyzed using the PyMOL software (Schrodinger; LLC, New York, NY, USA). For visual preferences, subunits have been deliberately removed from visualization in cases. For calculation of surface potential, Poisson-Boltzmann equation with appropriate Debye-Huckel length was solved using APBS electrostatic [22]. Calculation grid of 0.4, test solvent radius of 1.4 Å, solvent-accessible surface option was applied.
For recordings of the TRPC channels, we used NT solution and Cs+-rich solution. The NT solution contained 135 mM NaCl, 5 mM KCl, 2 mM CaCl2, 1 mM MgCl2, 10 mM glucose, and 10 mM HEPES with pH of 7.4 adjusted with NaOH. The Cs+-rich external solution contained equimolar CsCl instead of NaCl and KCl with pH adjusted to 7.4 using CsOH. The internal solution contained 140 mM CsCl, 10 mM HEPES, 0.2 mM Tris-GTP, 0.5 mM EGTA, and 3 mM Mg-ATP with pH of 7.3 adjusted with CsOH and used 0.2 mM GTPγS. All reagents were purchased from Sigma-Aldrich (St Louis, MO, USA).
In order to investigate the effect of polyamines to TRPC4 channel, we expressed mouse TRPC4b tagged with EGFP at the C-terminus in HEK293 cells. After a whole-cell configuration was achieved, Cs+-rich solution ([Cs+]o = 140 mM) was applied to magnify the activity of TRPC4 channel. Since TRPC4 channel can be activated by either infusion of intracellular GTPγS or stimulation of G protein-coupled receptors, we infused 0.2 mM of GTPγS inside pipette solution.
First, D629A mutant form of TRPC4 was expressed instead of wild-type channel. I–V curve of the channel showed outward-rectifying shape rather than doubly-rectifying shape in voltage range of −100 mV to +100 mV (Fig. 1A). Prominent decrease of inward current was in line with previous findings in TRPC5 channel [18]. Next, we added 1 mM of spermine in pipette solution and measured whole-cell current of D629A mutant of mouse TRPC4 channels. Outward current at +100 mV (I+100mV) was 36.13 ± 6.77 pA/pF in the absence of spermine whereas I+100mV was 17.48 ± 4.52 pA/pF when spermine was added in intracellular side. Inhibition of spermine in ratio (Ispermine(+)/Ispermine(−)) was 48.37 ± 25.03%. In wild-type TRPC4 channel, spermine inhibited I+100mV 8.26 ± 6.50%. The results above suggest that D629 residue contributes to blocking action of intracellular spermine.
Previously, we have found that glutamate residue at 728th and 729th position (E728 and E729) are responsible for interaction between TRPC4 channel and intracellular spermine [20]. Therefore, we examined whether D629A mutation in addition to truncation of amino acids from 720th position to 740th position is sufficient for recovering I+100mV from inhibition by spermine (Fig. 1B). Surprisingly, harboring both D629A mutation and truncation mutation yielded even larger inhibition by spermine. I+100mV of D629A/Δ(720–740) channel was 196.65 ± 87.39 pA/pF and 10.51 ± 4.16 pA/pF when intracellular spermine was introduced. We expanded deletion region even to 820th position if the recovery can be accomplished (Fig. 1C). In D629A/Δ(720–820) channel, I+100mV was 80.19 ± 16.45 pA/pF and 7.82 ± 2.05 pA/pF with intracellular spermine. Inhibitory effect of spermine to three mutated channels in relative scale shows that neither channels show promising recovery effect (Fig. 1D) except for partial recovery of D629A mutant.
Since no candidates fully recovered outward current, we surmised that there could be another binding sites responsible for channel-spermine interaction. From the result from D629A/Δ(720–820) truncated channels, it seemed obvious that Cterminal residues distal to 720th position was not promising, which leaves two options. First, acidic residues proximal to 629th position, which constructs central ion conducting pathway, can be a possible candidate. Second, acidic residues between 629th position and 720th position can be another candidate. In order to evaluate which option is more favorable, we examined if there exist any difference in the degree of inhibition depending on maximum conductance of the channel.
As a next step, we tried to apply notion of IRK in TRPC4 channels. Since TRPC4 channels are non-selective cation channels, we tested degrees of spermine block by increasing channel conductance with different activation systems but not by altering extracellular cation concentration. Previously, we have reported that alpha subunit of inhibitory subtype of heterotrimeric G-protein (Gαi) is strong activator of TRPC4 channels. Moreover, (−)-Englerin A, a selective TRPC channel agonist, was recently found [9]. Hence, we evaluated whether increasing conductance by such activation systems show different magnitude of current block by same concentration of spermine.
When gain-of-function mutant of Gαi2 protein was co-expressed with mouse TRPC4β channels, I+100mV of 325.32 ± 83.87 pA/pF was obtained (Fig. 2A). When 100 nM of (−)-Englerin A was applied in extracellular bath solution, I+100mV of 300.24 ± 136.93 pA/pF was measured (Fig. 2C). In both cases, normalized conductance curve over membrane potential showed typical N-shape and inactivation curve fitted to Boltzmann equation showed mean half-maximal voltage (V1/2) of −14.94 mV and −0.49 mV, respectively (Fig. 2B, D). Next, we analyzed how the intracellular infusion of 1 mM spermine changes whole-cell current of TRPC4 channels in both cases. In Gαi2 co-expression system, intracellular spermine reduced I+100mV to 77.69 ± 59.49 pA/pF. In (−)-Englerin A, I+100mV was reduced to 17.16 ± 0.64 mV (Fig. 2E). Half-maximal voltage of inactivation curve was left shifted in both cases to −32.82 mV and −16.8 mV, respectively. Lastly, inhibitory effect of spermine in current ratio showed that there is no correlation between magnitude of inhibitory action of spermine and magnitude of maximum current of the channel (Fig. 2F). The results above suggest that the chance is low of finding negative residues responsible for spermine binding inside central ion conducting pathway. Therefore, we investigated residues in between position 629 and 720, leaving residues proximal to 629th position behind.
From amino acid sequence map of mouse TRPC4 channels, we identified 5 acidic residues, namely, E648, E649, E687, E698 and E708. From the backbone of D629/Δ(720–740) channel, we added point mutations directed to the 5 sites above. All 5 channels showed doubly-rectifying I–V curves, implying that channel function was not changed from the mutation (Fig. 3A–E). Among five mutants, relative current shows that D629A/E649A/Δ(720–740) and D629A/E687A/Δ(720–740) channels show greatest current recovery. The results above suggest that residues E649, E687 contribute to blocking action of intracellular spermine.
In this study, we have shown that D629, a homologous site to D633 of TRPC5, is partially responsible for channel block by intracellular spermine. It is important to note, however, that current amplitude was greatly reduced in D629 mutant compared to wildtype channel. This adversary effect of mutation was previously reported in TRPC5 channels [18]. As in TRPC5, intracellular Mg2+ binding to D629 seems to somehow stabilize the entire channel structure or at least pore regions.
By co-expressing Gαi2 proteins and by using extracellular (−)-Englerin A, we deliberately increased maximum conductance of TRPC4 channels. This manipulation was to mimic high extracellular potassium concentration in IRK [1617]. In IRK, high extracellular potassium concentration decreased the magnitude of inward rectification. High extracellular potassium concentration made pore crowded with K+ ions and occupied most of negative residues inside the pore which is the site spermine bind with. We assumed that increased channel conductance would reflect increased ionic activity inside the pore, hence used Gαi2 and (−)-Englerin A. The experiment showed that there is no correlation between maximum channel conductance and magnitude of blocking action of intracellular spermine. It is noteworthy though, that either (−)-Englerin A group or Gαi2 group shows deviation from linear correlation (Fig. 2F). In other words, the inhibition of I+100mV in (−)-Englerin A group may have been exceptionally strong ((1) in Fig. 2F) or the inhibition in Gαi2 group may have been exceptionally weak ((2) in Fig. 2F), provided that linear correlation between % inhibition by spermine and I+100mV is true. The latter case is in special interest since recently revealed Cryo-EM structure of mouse TRPC4 channel shows that Gαi2 protein interacts with TRPC4 channel via rib helix (or connecting helix) of the channel. Previously, we have reported that negative amino acids in 720–740 region is crucial for spermine-mediated inward rectification of TRPC4 channel [20]. Interestingly, the 720–740 region exactly coincides with transition region between C-terminal rib helix and C-terminal coiled-coil domain (Fig. 4E). Although we cannot exclude the possibility that spermine and Gαi2 protein compete each other for binding to 720–740 region, electrophysiology so far suggests that there is little possibility that binding of Gαi2 to 720–740 region prohibits the binding of spermine to the same region, and vice versa. Spermine may bind to 720–740 region in medial fashion – by approaching inside the inner vestibule towards channel periphery – while Gαi2 protein bind to the same region in lateral fashion – by approaching the channel periphery from cytosolic space.
In the present study and from the previous ones [20], amino acids turned out to be important for spermine binding are D629, D632, E649, E687 and residues in 720–740 region. We have constructed structural image of those residues based on Cryo-EM data of mouse TRPC4 channel (Fig. 4A–E). Among those, D629, D632 and residues in 720–740 region line with contour of exceptionally large inner vestibule of TRPC4 channels. In contrast, E648 or E649 line with pre-S1 elbow and TRP helix, parallel to intracellular borderline of plasma membrane. Distinct location of E648, E649 and rest of the residues can be more clearly seen when the residues are merged together in tetrameric form (Fig. 5A). Based on structural information above, we hypothesized that E648 or E649 may act as peripheral attractor whose role is to reduce access resistance of spermine. Access resistance here describes total energetic barrier spermine may experience in changing partition – from cytosolic partition to inner vestibule partition. Surface potential analysis showed that areas corresponding to E648, E649 have significantly low potentials. Moreover, local potentials surrounding the area have highly positive values, creating local potential minimum with respect to possible reaction coordinates. Hence, E648 or E649 could lower the access resistance for spermine.
Lack of correlation between maximum conductance and magnitude of spermine inhibition not only supported the search to the residues in between 629th position and 720th position, but also decreased the likelihood of finding spermine binding sites inside the central pore. Although there could be lots of factors for explanation such as lack of acidic residues inside the pore, inappropriate distance among acidic residues, or many others, the absence of energetic stabilization for successful spermine-channel binding inside the pore would be the most critical. Unlike the case of inwardly-rectifying potassium channels with many acidic residues which is favorable for spermine binding, TRPC4 channel seems not to have such pore architecture. In other words, all binding actions of spermine projected to inward-rectification may be occurred solely on inner vestibule but not inside central ion conducting pathway (Fig. 5B). This finding may explain stark difference between IRK and TRPC4 channel: outward current burst at highly positive potentials. In TRPC4 channels, intracellular spermine binds with acidic residues such as D629, D632, E728, E729 or E687 and resides inside inner vestibule when moderate depolarization is applied (−60 mV to 40 mV). When membrane potential is highly depolarized (above 40 mV), monovalent cations responsible for outward current will flush into inner vestibule of the channel. A sudden increase of local potential in inner vestibule would push spermine away from the inner vestibule. At this point, the pathway spermine once used for changing partition (from Qcytosol to Qvestibule) may be used once again as an energetically favorable coordinate, leaving inner vestibule capacious. Spermine-free inner vestibule would be filled with charge-carrying cations, hence increased local ionic activity and sudden burst of outward current.
Polyamines are known for regulating proliferation, differentiation, would healing, scar formation [23]. In general, tissues with high proliferation rate have higher polyamine concentration levels. In gastrointestinal smooth muscle, it has been reported that extracellular polyamines inhibit mIcat [19]. The concentration of polyamines in intestinal lumen is in mM range [24] while intracellular concentrations of polyamines measured in guinea-pig ileum is 18, 206, and 385 µM for putrescine, spermidine, and spermine, respectively [25]. Intracellular polyamines lowered intracellular calcium concentration and contractility [262728] most likely by inhibiting voltage-gated calcium channels [262930]. Since channels responsible for mIcat—TRPC4 and TRPC6—show strong nonselective cationic inward current, they can contribute to membrane depolarization once opened. Consequently, the inhibition of mIcat by polyamines may reduce Ca2+ influx through voltage gated calcium channel. In guinea-pig ileal smooth muscle cells, 3 mM of extracellular spermine was sufficient to reduce mIcat to 50% [19]. The shape of I–V curve of reduced mIcat remained doubly-rectifying suggesting that inhibitory effect of extracellular spermine would be voltage-independent. In contrast, we have shown that intracellular spermine inhibits TRPC4 channel with strong voltage-dependency and micromolar (300 µM) concentration was sufficient to reduced TRPC4 current to 50% [20]. Since endogenous level of intracellular spermine in ileal smooth muscle is in similar range, spermine can be treated as tonic regulator of mIcat in the tissue.
Aforementioned membrane excitatory effect of mIcat may be partially attributed to non-selectivity among monovalent cations and subsequent depolarized reversal potential (~0 mV). Since activation kinetics of TRPC4 channel measured under voltage clamp is rather fast compared to action potential duration (APD) of GI smooth muscle, TRPC4 channel can act as an effective depolarizer, hence an action potential generator. More importantly, only with fully manifested inward rectification of TRPC4 channel can this peculiar excitatory effect of mIcat be achieved. Linear I–V curve would give strong outward cationic current at depolarized potentials and would shorten the APD.
Figures and Tables
 | Fig. 1Current densities of transient receptor potential canonical 4 (TRPC4) channels with different types of mutations.(A–C) Black dots represent current densities without spermine and red dots represent current densities with 1 mM intracellular spermine. (A) D629A mutation showed I+100mV of 36.13 ± 6.77 pA/pF and 17.48 ± 4.52 pA/pF when spermine was infused in pipette solution. (B) D629A/Δ(720–740) mutation showed I+100mV of 196.65 ± 87.39 pA/pF and 10.51 ± 4.16 pA/pF in the presence of intracellular spermine. (C) D629A/Δ(720–820) mutation showed I+100mV of 80.19 ± 16.45 pA/pF and 7.82 ± 2.05 pA/pF in the presence of intracellular spermine. (D) % inhibition of spermine calculated by current ratio between spermine (+) and spermine (−) groups.
|
 | Fig. 2Spermine blocks transient receptor potential canonical 4 (TRPC4) with similar degrees regardless of the channel's conductance.(A) Current densities of mouse TRPC4 channels co-expressed with Gαi2 proteins. (B) Normalized conductance curve of mouse TRPC4 channel coexpressed with Gαi2 proteins. Half-maximal voltage was −14.94 mV in the absence of spermine and −32.82 mV in the presence of 1 mM intracellular spermine. (C) Current densities of mouse TRPC4 channels activated by 100 nM of extracellular (−)-Englerin A. (D) Normalized conductance curve of mouse TRPC4 channel activated by 100 nM of extracellular (−)-Englerin A. Half-maximal voltage was −0.49 mV in the absence of spermine and −16.8 mV in the presence of intracellular spermine. For (A–D), black dots/lines represent current densities/Boltzmann fit without spermine and red dots/lines represent current densities/Boltzmann fit with intracellular spermine. Gray lines show normalized conductance of each group. (E) Current densities of each activation system at +100 mV and −100 mV. (F) Magnitude of I+100mV inhibition by spermine with respect to maximum I+100mV at each activation group.
|
 | Fig. 3Glutamate 649th and 687th are responsible for intracellular spermine binding.(A–E) Current densities of mutant channels. (A) D629A/E648A/Δ(720–740), (B) D629A/E649A/Δ(720–740), (C) D629A/E687A/Δ(720–740), (D) D629A/E698A/Δ(720–740), (E) D629A/E708A/Δ(720–740). (F) % inhibition of spermine calculated by current ratio between 1 mM spermine (+) and spermine (−) groups.
|
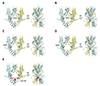 | Fig. 4Amino acid notations based on Cryo-EM structure of mouse transient receptor potential canonical 4 (TRPC4) channel.(A) D629, (B) D632, (C) E648, (D) E649, (E) residues from 720-740. For visual preferences, subunits in front of and behind of the screen was omitted on purpose. Each residue was colored red.
|
 | Fig. 5Structure of tetrameric transient receptor potential canonical 4 (TRPC4) and its putative spermine binding sites.(A) Upper panel; residues in Fig. 4 was all highlighted with red sticks. Yellow helices represent pore-helices (504–626), green helices represent TRP helix (634–658) and cyan helices represent C-terminal helix (696–753). Lower panel; surface potential of TRPC4 channel. For detailed information regarding calculation, please see Methods. Local potential minimum created by E648, E649 are highlighted with black arrow. (B) Model for blocking action of intracellular spermine in inwardly rectifying potassium channel (IRK) and TRPC4 channel.
|
ACKNOWLEDGEMENTS
This research was supported by the National Research Foundation of Korea, which is funded by the Ministry of Science, ICT (Information & Communication Technology), and Future Planning (MSIP) of the Korean government (2018R1A4A1023822 to I.S.) and by the Education and Research Encouragement Fund of Seoul National University Hospital (I.S.). We thank Jungeun Lee for carefully revising the manuscript.
Notes
Author contributions J.S.K., S.H.M., and T.W.K. performed electrophysiological experiments and analyzed data. J.S.K. designed study, generated figures, wrote the manuscript. J.Y.K. and Y.K.J. edited the paper. Y.C.S. provided technical supports. J.H.J. and I.S.S. provided the overall experimental advice and coordinated the study. All authors reviewed the manuscript.
References
3. Kim YC, Kim SJ, Sim JH, Cho CH, Juhnn YS, Suh SH, So I, Kim KW. Suppression of the carbachol-activated nonselective cationic current by antibody against alpha subunit of Go protein in guinea-pig gastric myocytes. Pflugers Arch. 1998; 436:494–496.

4. Sakamoto T, Unno T, Kitazawa T, Taneike T, Yamada M, Wess J, Nishimura M, Komori S. Three distinct muscarinic signalling pathways for cationic channel activation in mouse gut smooth muscle cells. J Physiol. 2007; 582(Pt 1):41–61.



5. Tsvilovskyy VV, Zholos AV, Aberle T, Philipp SE, Dietrich A, Zhu MX, Birnbaumer L, Freichel M, Flockerzi V. Deletion of TRPC4 and TRPC6 in mice impairs smooth muscle contraction and intestinal motility in vivo. Gastroenterology. 2009; 137:1415–1424.


6. Freichel M, Tsvilovskyy V, Camacho-Londoño JE. TRPC4- and TRPC4-containing channels. Handb Exp Pharmacol. 2014; 222:85–128.

7. Kim J, Ko J, Myeong J, Kwak M, Hong C, So I. TRPC1 as a negative regulator for TRPC4 and TRPC5 channels. Pflugers Arch. 2019; 471:1045–1053.


8. Kim J, Kwak M, Jeon JP, Myeong J, Wie J, Hong C, Kim SY, Jeon JH, Kim HJ, So I. Isoform- and receptor-specific channel property of canonical transient receptor potential (TRPC)1/4 channels. Pflugers Arch. 2014; 466:491–504.


9. Jeon JP, Hong C, Park EJ, Jeon JH, Cho NH, Kim IG, Choe H, Muallem S, Kim HJ, So I. Selective Gαi subunits as novel direct activators of transient receptor potential canonical (TRPC)4 and TRPC5 channels. J Biol Chem. 2012; 287:17029–17039.



10. Akbulut Y, Gaunt HJ, Muraki K, Ludlow MJ, Amer MS, Bruns A, Vasudev NS, Radtke L, Willot M, Hahn S, Seitz T, Ziegler S, Christmann M, Beech DJ, Waldmann H. (-)-Englerin A is a potent and selective activator of TRPC4 and TRPC5 calcium channels. Angew Chem Int Ed Engl. 2015; 54:3787–3791.


11. Lopatin AN, Makhina EN, Nichols CG. Potassium channel block by cytoplasmic polyamines as the mechanism of intrinsic rectification. Nature. 1994; 372:366–369.


12. Lopatin AN, Makhina EN, Nichols CG. The mechanism of inward rectification of potassium channels: “long-pore plugging” by cytoplasmic polyamines. J Gen Physiol. 1995; 106:923–955.



13. Stanfield PR, Davies NW, Shelton PA, Sutcliffe MJ, Khan IA, Brammar WJ, Conley EC. A single aspartate residue is involved in both intrinsic gating and blockage by Mg2+ of the inward rectifier, IRK1. J Physiol. 1994; 478(Pt 1):1–6.


14. Nishida M, MacKinnon R. Structural basis of inward rectification: cytoplasmic pore of the G protein-gated inward rectifier GIRK1 at 1.8 A resolution. Cell. 2002; 111:957–965.

15. Hibino H, Inanobe A, Furutani K, Murakami S, Findlay I, Kurachi Y. Inwardly rectifying potassium channels: their structure, function, and physiological roles. Physiol Rev. 2010; 90:291–366.


16. Pearson WL, Nichols CG. Block of the Kir2.1 channel pore by alkylamine analogues of endogenous polyamines. J Gen Physiol. 1998; 112:351–363.



17. Spassova M, Lu Z. Coupled ion movement underlies rectification in an inward-rectifier K+ channel. J Gen Physiol. 1998; 112:211–221.


18. Obukhov AG, Nowycky MC. A cytosolic residue mediates Mg2+ block and regulates inward current amplitude of a transient receptor potential channel. J Neurosci. 2005; 25:1234–1239.


19. Tsvilovskyy VV, Zholos AV, Bolton TB. Effects of polyamines on the muscarinic receptor-operated cation current in guinea-pig ileal smooth muscle myocytes. Br J Pharmacol. 2004; 143:968–975.



20. Kim J, Moon SH, Shin YC, Jeon JH, Park KJ, Lee KP, So I. Intracellular spermine blocks TRPC4 channel via electrostatic interaction with C-terminal negative amino acids. Pflugers Arch. 2016; 468:551–561.


21. Duan J, Li J, Zeng B, Chen GL, Peng X, Zhang Y, Wang J, Clapham DE, Li Z, Zhang J. Structure of the mouse TRPC4 ion channel. Nat Commun. 2018; 9:3102.



22. Jurrus E, Engel D, Star K, Monson K, Brandi J, Felberg LE, Brookes DH, Wilson L, Chen J, Liles K, Chun M, Li P, Gohara DW, Dolinsky T, Konecny R, Koes DR, Nielsen JE, Head-Gordon T, Geng W, Krasny R, et al. Improvements to the APBS biomolecular solvation software suite. Protein Sci. 2018; 27:112–128.

23. Casero RA Jr, Marton LJ. Targeting polyamine metabolism and function in cancer and other hyperproliferative diseases. Nat Rev Drug Discov. 2007; 6:373–390.


24. Osborne DL, Seidel ER. Gastrointestinal luminal polyamines: cellular accumulation and enterohepatic circulation. Am J Physiol. 1990; 258(4 Pt 1):G576–G584.

25. Swärd K, Nilsson BO, Hellstrand P. Polyamines increase Ca2+ sensitivity in permeabilized smooth muscle of guinea pig ileum. Am J Physiol. 1994; 266(6 Pt 1):C1754–C1763.
26. Gomez M, Hellstrand P. Effects of polyamines on voltage-activated calcium channels in guinea-pig intestinal smooth muscle. Pflugers Arch. 1995; 430:501–507.


27. Nilsson BO, Hellstrand P. Effects of polyamines on intracellular calcium and mechanical activity in smooth muscle of guinea-pig taenia coli. Acta Physiol Scand. 1993; 148:37–43.


28. Onodera K, Unemoto T, Miyaki K, Hayashi M. Pharmacological studies on polyamines. I. Relaxing effect of spermine and spermidine on smooth muscle of guinea pig ileum contracted by 5-hydroxytryptamine and nicotine. Arch Int Pharmacodyn Ther. 1968; 174:491–494.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download