Abstract
Purpose
5-Alpha reductase inhibitors (5ARI), inhibit the conversion of testosterone to dihydrotestosterone, which is essential in prostate hyperplasia, and decreases the prostate volume directly. On the other hand, 5ARI have a range of side effects, such as sexual dysfunction. After the discontinuation of 5ARI, prostate regrowth occurs rapidly until it reaches the baseline size. This study examined the effects of 5ARI when used intermittently.
Materials and Methods
Between March 2009 and May 2017, patients who visited one physician's outpatient clinic and were diagnosed with BPH underwent transrectal ultrasonography. The selected patients began to take 5ARI until the prostate size decreased at least 10% of the baseline (the first medication). After confirming adequate prostate shrinkage, the patients stopped medication until prostate regrowth reached 50% of the decreased size. After regrowth, they restarted medication for one year (second medication). The prostate size, serum prostate specific antigen (PSA) levels, international prostate symptom score (IPSS) scores, and maximum flow rate (Qmax) in uroflowmetry were collected after the first and second medication and compared using paired t-tests.
Results
Sixty patients with a mean age of 65.1 years were included in the study. The prostate size and serum PSA level increased after the second medication compared to the first, and the prostate reduction and Qmax in uroflowmetry decreased significantly. On the other hand, the symptoms felt by the patients surveyed by the IPSS scores showed no significant difference.
Go to : 
References
1. Boyle P, Roehrborn C, Harkaway R, Logie J, de la Rosette J, Emberton M. 5-Alpha reductase inhibition provides superior benefits to alpha blockade by preventing AUR and BPH-related surgery. Eur Urol. 2004; 45:620–6. ; discussion 626–7.

2. Jacobsen SJ, Jacobson DJ, Girman CJ, Roberts RO, Rhodes T, Guess HA, et al. Natural history of prostatism: risk factors for acute urinary retention. J Urol. 1997; 158:481–7.

3. Oelke M, Bachmann A, Descazeaud A, Emberton M, Gravas S, Michel MC, et al. Guidelines on the management of male lower urinary tract symptoms (LUTS), incl. benign prostatic obstruction (BPO). Arnhem: European Association of Urology;2013.
4. Marks LS, Andriole GL, Fitzpatrick JM, Schulman CC, Roehrborn CG. The interpretation of serum prostate specific antigen in men receiving 5alpha-reductase inhibitors: a review and clinical recommendations. J Urol. 2006; 176:868–74.
5. Seftel AD, de la Rosette J, Birt J, Porter V, Zarotsky V, Viktrup L. Coexisting lower urinary tract symptoms and erectile dysfunction: a systematic review of epidemiological data. Int J Clin Pract. 2013; 67:32–45.

6. Kim W, Jung JH, Kang TW, Song JM, Chung HC. Clinical effects of discontinuing 5-alpha reductase inhibitor in patients with benign prostatic hyperplasia. Korean J Urol. 2014; 55:52–6.

7. Choi K, Kim B, Cho IC, Min SK. Patient's factors correlated with prostate volume recovery after 5 alpha reductase inhibitor discontinuation. Urogenit Tract Infect. 2018; 13:79–83.

8. Girman CJ. Population-based studies of the epidemiology of benign prostatic hyperplasia. Br J Urol. 1998; 82(Suppl 1):34–43.

9. Emberton M, Andriole GL, de la Rosette J, Djavan B, Hoefner K, Vela Navarrete R, et al. Benign prostatic hyperplasia: a progressive disease of aging men. Urology. 2003; 61:267–73.

10. Shin YS, Lee JW, Kim MK, Jeong YB, Park SC. Early dutasteride monotherapy in men with detectable serum prostate-specific antigen levels following radical prostatectomy: a prospective trial. Investig Clin Urol. 2017; 58:98–102.

11. Kirby RS, Roehrborn C, Boyle P, Bartsch G, Jardin A, Cary MM, et al. Prospective European Doxazosin and Combination Therapy Study Investigators. Efficacy and tolerability of doxazosin and finasteride, alone or in combination, in treatment of symptomatic benign prostatic hyperplasia: the Prospective European Doxazosin and Combination Therapy (PREDICT) trial. Urology. 2003; 61:119–26.

12. Djavan B, Marberger M. A metaanalysis on the efficacy and tolerability of alpha1-adrenoceptor antagonists in patients with lower urinary tract symptoms suggestive of benign prostatic obstruction. Eur Urol. 1999; 36:1–13.
13. Kaplan SA, Lee JY, Meehan AG, Kusek JW. MTOPS Research Group. Long-term treatment with finasteride improves clinical progression of benign prostatic hyperplasia in men with an enlarged versus a smaller prostate: data from the MTOPS trial. J Urol. 2011; 185:1369–73.

14. Andersen JT, Nickel JC, Marshall VR, Schulman CC, Boyle P. Finasteride significantly reduces acute urinary retention and need for surgery in patients with symptomatic benign prostatic hyperplasia. Urology. 1997; 49:839–45.

15. Madersbacher S, Marszalek M, Lackner J, Berger P, Schatzl G. The longterm outcome of medical therapy for BPH. Eur Urol. 2007; 51:1522–33.

16. Nickel JC, Fradet Y, Boake RC, Pommerville PJ, Perreault JP, Afridi SK, et al. Efficacy and safety of finasteride therapy for benign prostatic hyperplasia: results of a 2-year randomized controlled trial (the PROSPECT study). PROscar Safety Plus Efficacy Canadian Two Year Study. CMAJ. 1996; 155:1251–9.
17. Hudson PB, Boake R, Trachtenberg J, Romas NA, Rosenblatt S, Narayan P, et al. Efficacy of finasteride is maintained in patients with benign prostatic hyperplasia treated for 5 years. The North American Finasteride Study Group. Urology. 1999; 53:690–5.
18. Corona G, Tirabassi G, Santi D, Maseroli E, Gacci M, Dicuio M, et al. Sexual dysfunction in subjects treated with inhibitors of 5-reductase for benign prostatic hyperplasia: a comprehensive review and metaanalysis. Andrology. 2017; 5:671–8.

19. Trost L, Saitz TR, Hellstrom WJ. Side effects of 5-alpha reductase inhibitors: a comprehensive review. Sex Med Rev. 2013; 1:24–41.

20. Stoner E. The clinical effects of a 5 alpha-reductase inhibitor, finasteride, on benign prostatic hyperplasia. The Finasteride Study Group. J Urol. 1992; 147:1298–302.
21. Jeong YB, Kwon KS, Kim SD, Kim HJ. Effect of discontinuation of 5alpha-reductase inhibitors on prostate volume and symptoms in men with BPH: a prospective study. Urology. 2009; 73:802–6.
Go to : 
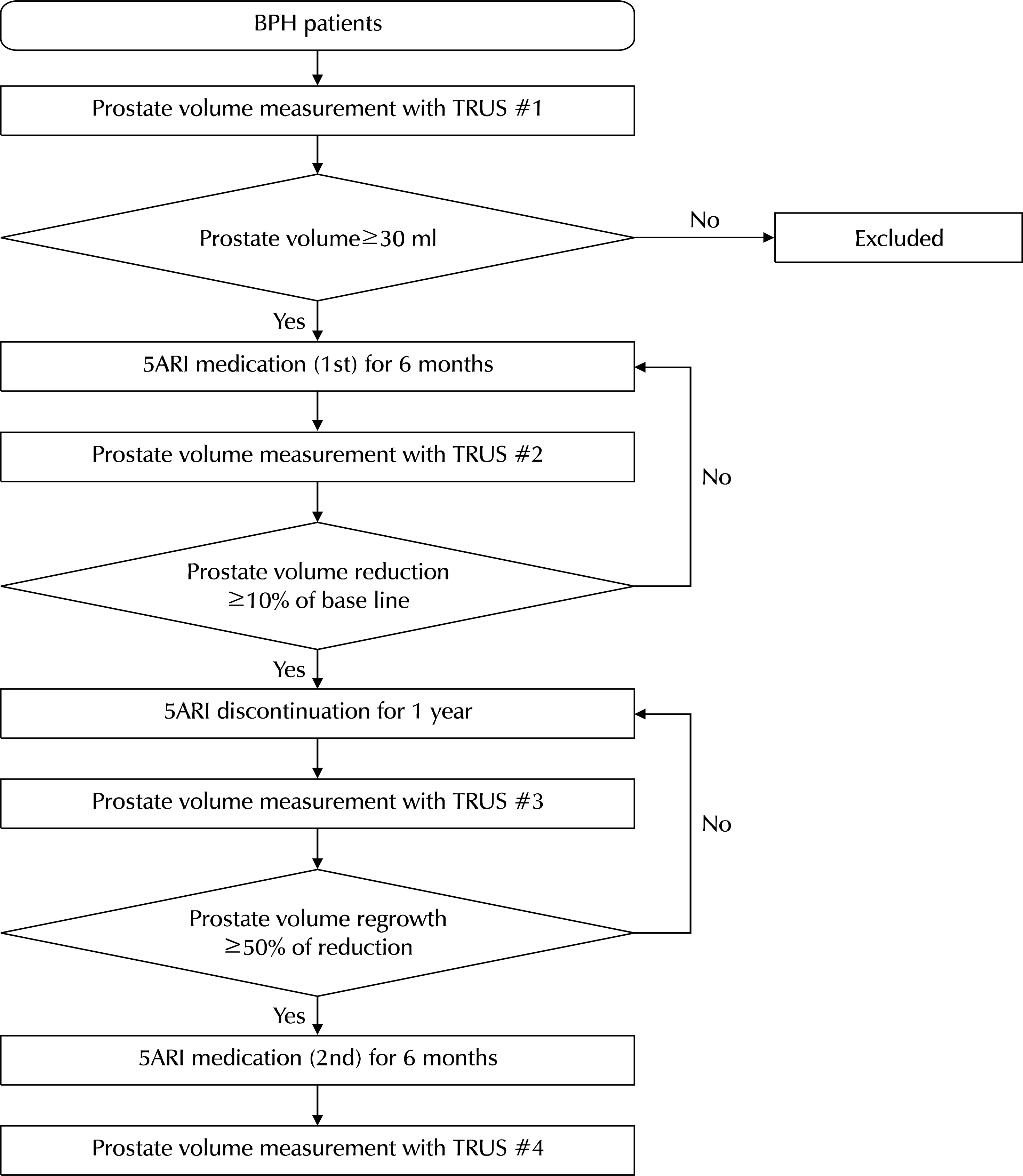 | Fig. 1.Flow chart of the study course and patient selection. BPH: benign prostate hyperplasia, TRUS: transrectal ultrasonography, 5ARI: 5-alpha reductase inhibitors. |
Table 1.
Changes in the clinical features during the 1st medication, discontinuation, and 2nd medication (n=60)
Table 2.
Results of statistical analysis between 1st and 2nd medication




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download