Abstract
Purpose
As a minimal invasive device for benign prostatic hyperplasia (BPH) treatment, prostatic urethral lift (PUL) is widely accepted worldwide but not widely used in Korea. We investigated the one-year results of for patients with BPH in Korea.
Materials and Methods
From April 2017 to June 2018, 42 patients with BPH were treated with PUL under local anesthesia with sedation. International Prostate Symptom Score (IPSS) and maximum urinary flow rate and post-void residual (PVR) were evaluated preoperatively and 1, 3, 6, and 12 months later.
Results
Mean age was 69.57±8.58 years old, and mean prostatic volume was 37.17±12.19 cc. Preoperative total IPSS and quality of life (QOL) were 19.94±7.81 and 3.69±1.30, respectively. Total IPSS improved to 11.26±7.22 (p<0.001), and QOL was 2.42±1.43 (p=0.01) after one month. Patients showed no evidence of inflammation related to the implants. IPSS and QOL were somewhat worse after 3 months but were better than baseline at 6 and 12 months. Preoperative maximum flow rate (Qmax) was 9.71±5.45 ml/sec, and one month after surgery, it had improved to 12.63±7.33 (p=0.01); it remained good at 3, 6, and 12 months (12.63±7.38, 12.45±7.39, 14.73±9.67). PVR was not significant at any points postoperative (80.61±67.91 to 43.95±8.19, p=0.119). No patient reported retrograde ejaculation, erectile dysfunction or urinary tract infection.
Go to : 
References
1. Verhamme KM, Dieleman JP, Bleumink GS, Bosch JL, Stricker BH, Sturkenboom MC. Treatment strategies, patterns of drug use and treatment discontinuation in men with LUTS suggestive of benign prostatic hyperplasia: the Triumph project. Eur Urol. 2003; 44:539–45.

2. Roehrborn CG. Current medical therapies for men with lower urinary tract symptoms and benign prostatic hyperplasia: achievements and limitations. Rev Urol. 2008; 10:14–25.
3. Park HK, Park H, Cho SY, Bae J, Jeong SJ, Hong SK, et al. The prevalence of benign prostatic hyperplasia in elderly men in Korea: a community-based study. Korean J Urol. 2009; 50:843–7.

4. AUA Practice Guidelines Committee. AUA guideline on management of benign prostatic hyperplasia (2003). Chapter 1: diagnosis and treatment recommendations. J Urol. 2003; 170:530–47.
5. Nickel JC, Saad F. The American Urological Association 2003 guideline on management of benign prostatic hyperplasia: a Canadian opinion. Can J Urol. 2004; 11:2186–93.
6. McVary KT, Roehrborn CG, Avins AL, Barry MJ, Bruskewitz RC, Donnell RF, et al. Update on AUA guideline on the management of benign prostatic hyperplasia. J Urol. 2011; 185:1793–803.

7. Bouza C, López T, Magro A, Navalpotro L, Amate JM. Systematic review and metaanalysis of transurethral needle ablation in symptomatic benign prostatic hyperplasia. BMC Urol. 2006; 6:14.

8. Andersen M, Walter S. [Microwave thermotherapy for benign prostatic hyperplasia. A survey of a Cochrane review]. Ugeskr Laeger. 2009; 171:2281–4. Danish.
9. Chin PT, Bolton DM, Jack G, Rashid P, Thavaseelan J, Yu RJ, et al. Prostatic urethral lift: two-year results after treatment for lower urinary tract symptoms secondary to benign prostatic hyperplasia. Urology. 2012; 79:5–11.

10. McNicholas TA, Woo HH, Chin PT, Bolton D, Fernández Arjona M, Sievert KD, et al. Minimally invasive prostatic urethral lift: surgical technique and multinational experience. Eur Urol. 2013; 64:292–9.

11. Jones P, Rai BP, Aboumarzouk OM, Somani BK. Urolift: a new chapter in benign prostate hyperplasia (BPH) therapy. Ann Transl Med. 2016; 4:116.

12. Jones P, Rajkumar GN, Rai BP, Aboumarzouk OM, Cleaveland P, Srirangam SJ, et al. Medium-term outcomes of urolift (minimum 12 months follow-up): evidence from a systematic review. Urology. 2016; 97:20–4.

13. Statistics Korea. Korean Statistical Information Service [Internet]. Daejeon: Statistics Korea;2014. [cited 2018 Oct 5]. Available from:. http://kosis.kr/index/index.do.
14. Lee YJ, Lee JW, Park J, Seo SI, Chung JI, Yoo TK, et al. Nationwide incidence and treatment pattern of benign prostatic hyperplasia in Korea. Investig Clin Urol. 2016; 57:424–30.

15. Barrack ER, Bujnovszky P, Walsh PC. Subcellular distribution of androgen receptors in human normal, benign hyperplastic, and malignant prostatic tissues: characterization of nuclear salt-resistant receptors. Cancer Res. 1983; 43:1107–16.
16. Martin DJ, Mulhall JP. Enlarging the scope of managing benign prostatic hyperplasia: addressing sexual function and quality of life. Int J Clin Pract. 2005; 59:579–90.

17. Ulchaker JC, Martinson MS. Cost-effectiveness analysis of six therapies for the treatment of lower urinary tract symptoms due to benign prostatic hyperplasia. Clinicoecon Outcomes Res. 2017; 10:29–43.

18. Roehrborn CG, Gange SN, Shore ND, Giddens JL, Bolton DM, Cowan BE, et al. The prostatic urethral lift for the treatment of lower urinary tract symptoms associated with prostate enlargement due to benign prostatic hyperplasia: the L.I.F.T. Study. J Urol. 2013; 190:2161–7.
19. Cantwell AL, Bogache WK, Richardson SF, Tutrone RF, Barkin J, Fagelson JE, et al. Multicentre prospective crossover study of the ‘prostatic urethral lift'for the treatment of lower urinary tract symptoms secondary to benign prostatic hyperplasia. BJU Int. 2014; 113:615–22.
20. Shore N, Freedman S, Gange S, Moseley W, Heron S, Tutrone R, et al. Prospective multicenter study elucidating patient experience after prostatic urethral lift. Can J Urol. 2014; 21:7094–101.
21. Rukstalis DB. Prostatic urethral lift: a novel approach for managing symptomatic BPH in the aging man. Can J Urol. 2015; 22(Suppl 1):67–74.
22. S⊘nksen J, Barber NJ, Speakman MJ, Berges R, Wetterauer U, Greene D, et al. Prospective, randomized, multinational study of prostatic urethral lift versus transurethral resection of the prostate: 12-month results from the BPH6 study. Eur Urol. 2015; 68:643–52.
23. Rassweiler J, Teber D, Kuntz R, Hofmann R. Complications of transurethral resection of the prostate (TURP)–incidence, management, and prevention. Eur Urol. 2006; 50:969–79. ; discussion 980.

24. Alloussi SH, Lang C, Eichel R, Alloussi S. Ejaculation-preserving transurethral resection of prostate and bladder neck: short-and longterm results of a new innovative resection technique. J Endourol. 2014; 28:84–9.
Go to : 
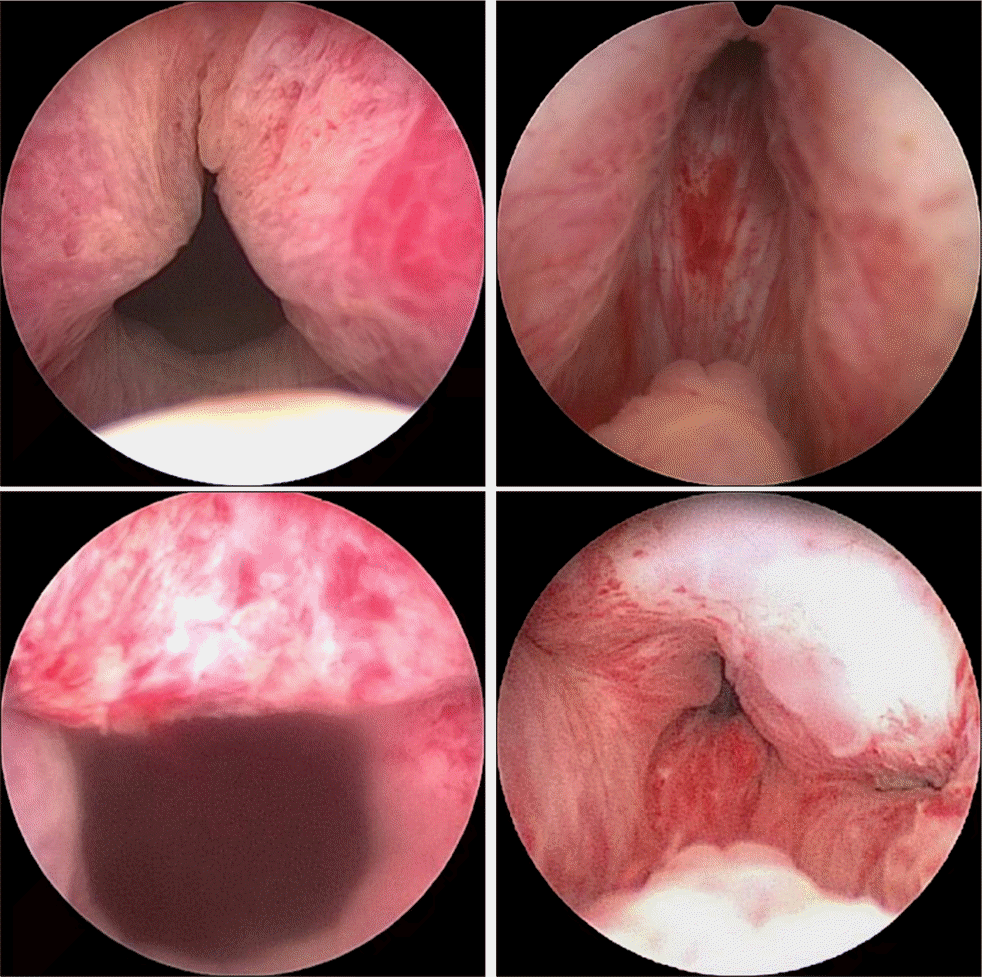 | Fig. 1.Cystoscopic features before (upper) and after (lower) prostatic urethral lift patients. |
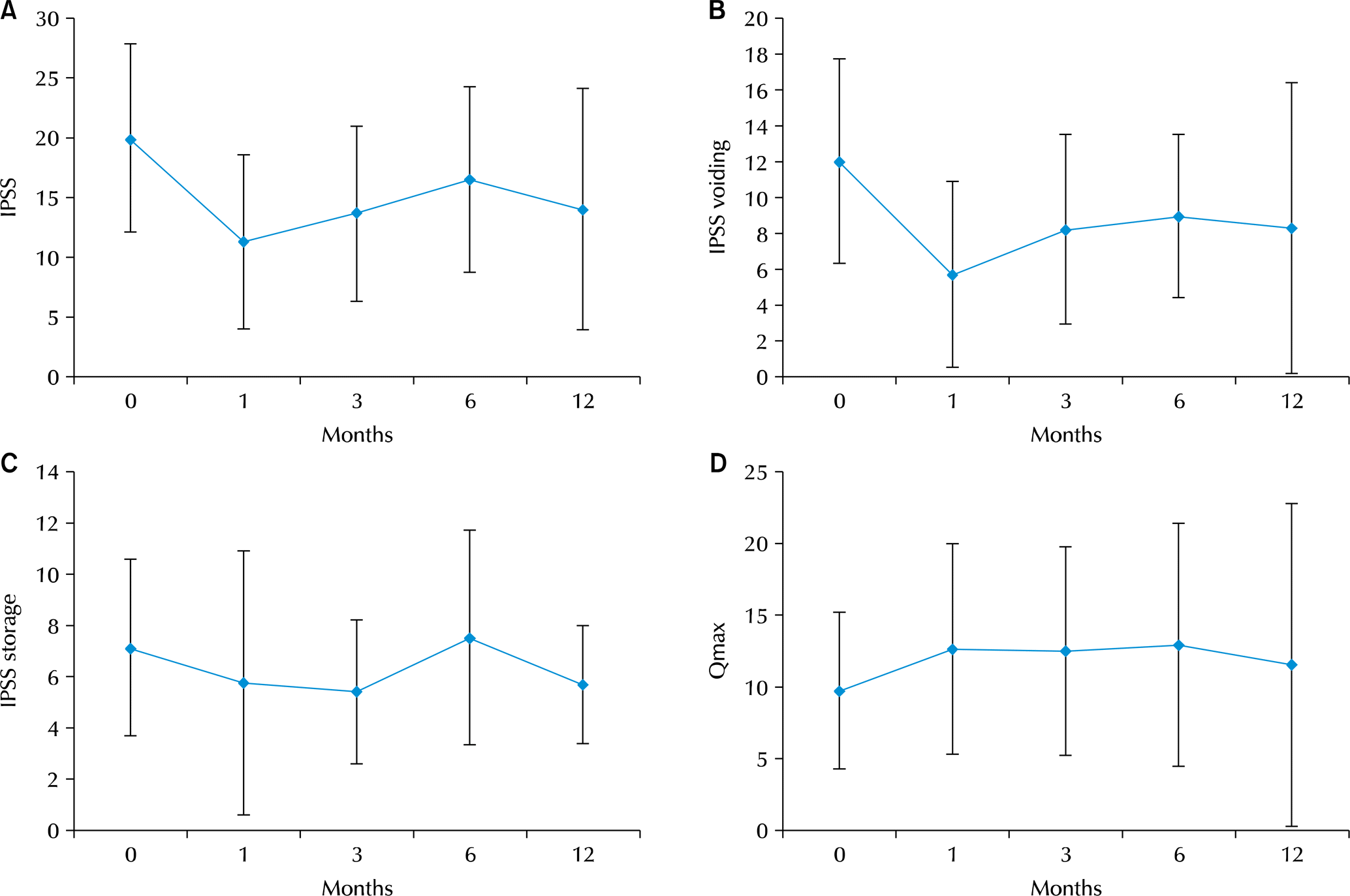 | Fig. 2.Preoperative, one, three, six, and 12 months results for prostatic urethral lift. (A) Total International Prostate Symptom Score (IPSS), (B) IPSS voiding score, (C) IPSS storage score, (D) maximum flow rate (Qmax). |
Table 1.
Subjects' demographics
Values are presented as mean±standard deviation or average (range). IPSS: International Prostate Symptom Score, QOL: quality of life, Qmax: maximum flow rate, PdetQmax: detrusor pressure at peak flow rate, BOOI: bladder outlet obstruction index, BCI: bladder contractility index, PVR: post-void residual.
Table 2.
Change in the outcomes after prostatic urethral lift: one month to 12 months
Table 3.
Overview of adverse events over one year after the prostatic urethral lift




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download