This article has been
cited by other articles in ScienceCentral.
Abstract
Purpose
The objective of this research was to examine the cold chain temperature maintenance for the supply of vaccines and other biological products by pharmaceutical wholesaler.
Materials and Methods
In this study, six configurations using cold vaccine boxes or bags made with different materials, with and without insulation, of different sizes, and number of coolant-packs were used to simulate the configuration used by the pharmaceutical wholesalers for transportation of vaccine. Model vaccines (vial, n=10) were packed using these six configurations which then stored in an incubator at 38℃ and monitored for 24 hours. Each configuration was tested repeatedly for 5 times.
Results
In term of compliance to 2℃–8℃, four out of six tested configurations are effective in cold chain transportation. The effectiveness is highly dependent on the type of passive containers used, size of cold boxes, insulation, and number of coolant-packs. The configuration with a larger polystyrene foam box with five coolant-packs maintained the required temperature up to 23 hours. In contrast, configurations using a polystyrene foam box with four coolant-packs and a large vaccine cold box with two coolant-packs failed to reach below 8℃ throughout the 24 hours.
Conclusion
Packaging method, the material and size of the container could have a direct impact on the effectiveness of cold chain temperature maintenance. Polystyrene foam box, cold box with polyethylene interior lining and polypropylene insulation, a cooler bag with proper number of ice packs could be effectively used for transportation of vaccines within their respective transportation duration allowance.
Go to :

Keywords: Cold chain management, Temperature, Pharmaceutical wholesaler, Transportation
Introduction
Vaccines are biological products may loss of potency or even destroyed with each episode of exposure to temperatures below or above the recommended temperature range (2℃–8℃) [
1]. To maintain vaccines perfectly conserved right from the manufacturing facility to patient use requires a satisfactory cold chain set-up, compliance to standards, and effective logistic management [
2]. The standard procedure of cold chain management should be adhered to the standard consistently whereby the supply chain cycle equipped with rigorous temperature and humidity control throughout the supply chain stages [
3]. Improper storage during transportation which may expose vaccines to temperatures outside the recommended ranges can decrease their potency [
4].
An accidental event in which a vaccine or other biological product is exposed to temperatures outside the ranges designated for transport and storage is termed as temperature excursion [
5]. Temperature excursion refers to keeping vaccines below the recommended temperature which can be as harmful as keeping them above that temperature range, since most vaccines may be damaged by freezing [
6]. Therefore, an effective vaccine supply chain and logistics system is crucial to safeguard product quality. One appropriate example is the Thailand case study where a vendor-managed inventory system was used to ensure full compliance of vaccine supply management and distribution [
7]. Common pitfalls are non-fully functioning refrigeration apparatus, non-compliance with cold-chain procedures, lack of understanding of the vaccine damage and inadequate monitoring practice [
89].
Vaccines, being biological products, have a limited shelf life and the potency of vaccines is guaranteed provided if the stock is transported and stored within the stipulated conditions. The optimum temperature for refrigerated vaccine is between 2℃ to 8℃ whereas for frozen vaccine the minimum temperature is −15℃. Once a vaccine has been thermally compromised, its loss of potency cannot be reversed [
12]. Good Distribution and Storage Practices clearly state that temperature-sensitive products should be stored, handled, and distributed with great care throughout the distribution network. This serves as a requirement of temperature monitoring throughout the whole supply chain process [
1011].
The common necessity of those product storage is the strict high requirements on the temperature, humidity, light, or other particular conditions [
12]. For example, since heat and direct sunlight are detrimental to the integrity of vaccine, the storing of vaccine should be in its original packaging until the time of administration. The term “passive container” was used by the World Health Organization (WHO) to describe “a container that maintains a temperature-controlled environment inside an insulated enclosure, generally without thermostatic regulation, using frozen, conditioned, cool, or warm coolant-packs” [
2]. Some examples of passive containers used in cold chain are vaccine carriers, reusable insulated cold boxes and single-use insulated cartons [
121314]. However, there is a gap in the current knowledge and practice on the proper usage of large capacity vaccine cold boxes [
14], vaccine cold boxes [
13], and carriers [
12] because the WHO Performance, Quality and Safety (PQS) catalogue does not list net storage capacity for cold boxes and carriers [
121314]. According to PQS catalogue, the gross volume can be calculated based on the values for internal dimensions of the vaccine compartment, taking into account of having coolant-packs in place [
2]. In this particular case, PQS catalogue only suggests the pharmaceutical wholesaler to load the vaccine carrier or cold box with the designated number of frozen coolant-packs [
2].
To the authors' best knowledge, no study has been reported before to simulate the real performance of cold chain from pharmaceutical wholesaler to their customers such as clinics, hospitals, and pharmacies. Thus, the objective of this study was to evaluate the performance of the packaging methods practised by the pharmaceutical wholesalers, in compliance with the PQS specification stipulated by WHO [
2121314]. This study also served to design a good packaging method for cold chain supply. The study examined various characteristics including the choice of packaging materials, number and arrangement of cold pack as well as duration of transportation.
Go to :

Materials and Methods
Study design and configurations
There were six configurations of vaccine packaging used for this study (
Table 1). For each configuration, a total of 10 vials of anti-tetanus vaccine (Tetanus toxoid vaccine adsorbed; 10 dose vials of 5.0 mL; Serum Institute of India, Pune, India) were. When the vaccines were not in use for experiments, the vials were stored between 2℃ to 8℃ in a refrigerator of which the temperature was closely monitored.
Table 1
The six configurations tested for the storage of anti-tetanus vaccine
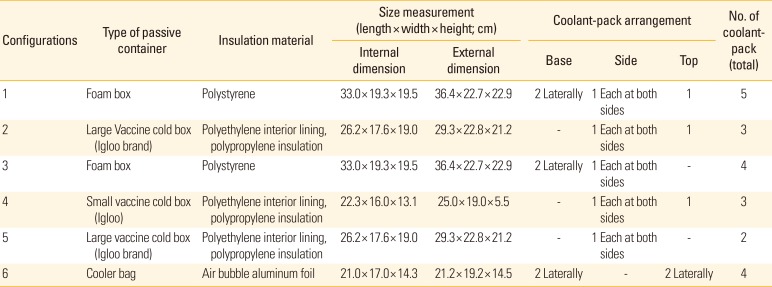
|
Configurations |
Type of passive container |
Insulation material |
Size measurement (length × width × height; cm) |
Coolant-pack arrangement |
No. of coolant- pack (total) |
|
Internal dimension |
External dimension |
Base |
Side |
Top |
|
1 |
Foam box |
Polystyrene |
33.0 × 19.3 × 19.5 |
36.4 × 22.7 × 22.9 |
2 Laterally |
1 Each at both sides |
1 |
5 |
|
2 |
Large Vaccine cold box (Igloo brand) |
Polyethylene interior lining, polypropylene insulation |
26.2 × 17.6 × 19.0 |
29.3 × 22.8 × 21.2 |
- |
1 Each at both sides |
1 |
3 |
|
3 |
Foam box |
Polystyrene |
33.0 × 19.3 × 19.5 |
36.4 × 22.7 × 22.9 |
2 Laterally |
1 Each at both sides |
- |
4 |
|
4 |
Small vaccine cold box (Igloo) |
Polyethylene interior lining, polypropylene insulation |
22.3 × 16.0 × 13.1 |
25.0 × 19.0 × 5.5 |
- |
1 Each at both sides |
1 |
3 |
|
5 |
Large vaccine cold box (Igloo brand) |
Polyethylene interior lining, polypropylene insulation |
26.2 × 17.6 × 19.0 |
29.3 × 22.8 × 21.2 |
- |
1 Each at both sides |
- |
2 |
|
6 |
Cooler bag |
Air bubble aluminum foil |
21.0 × 17.0 × 14.3 |
21.2 × 19.2 × 14.5 |
2 Laterally |
- |
2 Laterally |
4 |

The configuration 1, 2, 4, and 6 simulated the packaging methods used by pharmaceutical wholesalers whereas configuration 3 and 5 were designed to study the impact of different number of coolant-pack(s) used in each packaging. The temperature in each storage configuration was monitored for 24 hours, with each configuration was tested repeatedly for 5 times.
The size of the coolant-pack (Thermafreeze ice pack; Thermarite, Petaling Jaya, Malaysia) was 14.0×6.5 cm and weighted approximately 90.0 g when it was hydrated. Prior to use, the coolant-pack was immersed in water for 15 minutes to be fully hydrated and then placed in the freezer for a minimum of 24 hours to ensure it was fully frozen.
In order to ensure the outer packaging of the vaccine is not soiled and fully dry, the vaccines were protected using a simple and makeshift insulator. The insulator was made up from a low-density polyethylene plastic bag (12.0×16.0 cm) and newspaper. For each configuration, 10 vials with a data logger inserted into a low-density polyethylene plastic bag and opening was tied tightly. Then, the bag was then wrapped with two layers of newspaper.
Time and temperature recording
Each configuration (passive container with vaccine vials and data logger) was left in a large incubator with temperature set at a 38℃ simulating the temperature of transporting vehicle without air-conditioning. Data loggers were used to track the temperature changes inside the package of vaccines, when the vaccines are being sent to the customer. Two temperature and humidity USB data loggers (Alpha TH30; TempSen Electronics, Shanghai, China) which were newly calibrated and validated were used to record internal temperature inside the container and external temperature. The measurement range of the data logger was between −30℃ to 70℃ for temperature and 0%–100% for humidity level. The external data logger which was used to monitor the external temperature for 24 hours to simulate the harsh environment during transportation process. Another data logger that was used to monitor external temperature was placed next to vaccine container to record down the external temperature.
Statistical analysis: comparison of temperature-time profiles
The pair-wise statistical procedure using the difference factor (f1; Equation 1) and f1area were used to compare the temperature-time profiles obtained. The difference factor measures the percent error between two curves.
Where R
t and T
t are the cumulative percentage dissolved at each of the selected time points of the reference and test product. When the test and reference profiles are identical, the percent error is zero, and increases proportionately with an increase in dissimilarity between the two temperature-time profiles. Meanwhile, for difference factor f
1area, the difference of profiles is calculated based on Equation 2 [
16].
Where WR and WT are the reference and test profiles at time t, respectively. Evaluation of f1area was considered up to the time corresponding to time zero to time at 24 hours. In this project f1area was applied from mean data sets. For instance, a f1area=0.20 suggested 20% average difference of a test from a reference data set. For each temperature-time profile comparison, configuration 1 was chosen as the reference profile as configuration 1 is the most commonly practiced method.
Go to :

Results
The mean temperature (±standard deviation, n=5) values of internal temperature of each configuration over 24 hours are shown in
Fig. 1.
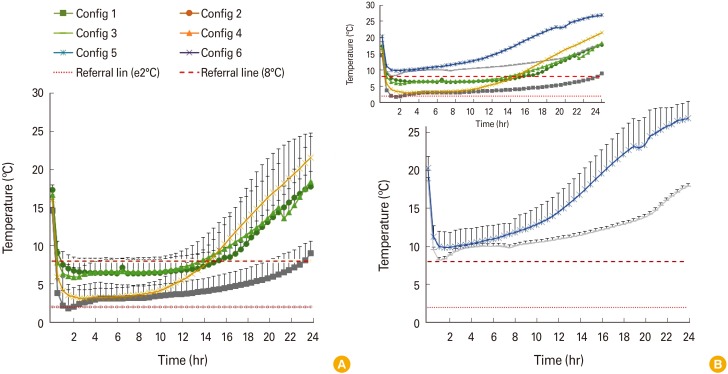 | Fig. 1(A, B) Mean temperature (±standard deviation, n=5) values of internal temperature of each configuration over 24 hours.
|
Among the configurations, configuration 1, using a polystyrene foam box with five coolant-packs, reached the temperature range of 2.02℃ to 7.54℃ and the temperature was maintained for 23 hours. This was the longest duration of maintenance time, in which the intended cold chain temperature range (2℃–8℃) was maintained compared to the rest of other configurations. Nonetheless, it was noted that configuration 2, using a large vaccine cold box with polyethylene interior lining, polypropylene insulation and three coolant-packs, recorded internal temperature ranges of 6.34℃ to 7.88℃ and lasted for 15 hours. The duration was 8 hours shorter compared to configuration 1. Besides, it was noted that configuration 2 required more than 30 minutes for the internal temperature to reach below 8℃. In contrast, for configuration 4, using a small vaccine cold box with polyethylene interior lining, polypropylene insulation and three coolant-packs, within 30 minutes, it reached the intended temperature, ranged 5.88℃–7.56℃ and maintained for 13.5 hours. Likewise, configuration 6, using a cooler bag, the smallest and lightest container among the type of passive containers used, with four coolant-packs recorded a temperature below 8℃ within 30 minutes after first exposure to external environment and maintained at 3.1℃–7.3℃, lasted up to 14.5 hours.
Meanwhile, both configuration 3 and 5 failed to reach below 8℃ throughout the 24 hours. Configuration 3, a polystyrene foam box with four coolant-packs, was a storage condition with only a missing a coolant-pack on top compared to configuration 1. The lowest temperature it managed to reach was 8.2℃ in first hour. Similarly, configuration 5, missing a coolant-pack on top compared to configuration 2, hit the lowest temperature only at 9.9℃ after 2 hours in the storage packing.
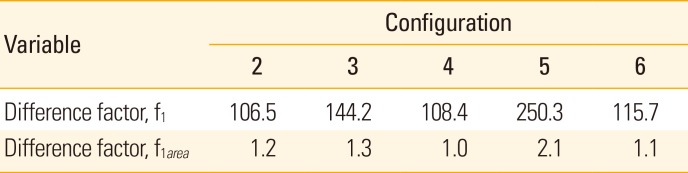
Table 2 shows the comparison of temperature-time profiles using pair-wise approach. Configuration 1 was used as reference profile for the comparison with the other five configurations. All had a more than 100% difference. Among the comparisons, configuration 5 had the highest differences from configuration 1, with f
1 value of 250.3 and f
1area of 2.1. In contrast, the temperature values of configuration 2, 4, and 6 compared to configuration 1 were relatively similar to each other, with 106.5, 108.4, and 115.7, respectively.
Table 2
The comparison of temperature-time profiles of five configurations with configuration 1
|
Variable |
Configuration |
|
2 |
3 |
4 |
5 |
6 |
|
Difference factor, f1
|
106.5 |
144.2 |
108.4 |
250.3 |
115.7 |
|
Difference factor, f1area
|
1.2 |
1.3 |
1.0 |
2.1 |
1.1 |

Go to :

Discussion
Time- and temperature-controlled storage and transport of vaccines in compliance of manufacturer specifications is essential to ensure optimal therapeutic effect of vaccine requiring cold chain [
517]. Ineffective or degraded vaccines could be part of the reason of resurgence of certain vaccine-preventable diseases [
18]. Degradation happens unintentionally especially for aluminum adjuvant–containing vaccines which are highly sensitive to unintentional freezing such as adjacent to frozen ice packs or refrigerator freezer coils [
19].
Even though a recent review of Vaccine Adverse Event Reporting System (VAERS) by Hibbs et al. [
5] indicated that the use of vaccines kept outside of recommended temperatures caused no substantial direct health risk, possible decreased protection is inevitable. Of note, with the benefit of hindsight, Shimabukuro et al. [
20] reported that VAERS data are not suitable to determine the possibility of an adverse event induced by a vaccine. It is speculated that the interpretation of VAERS data alone or out of clinical context could lead to erroneous conclusions about the risk of adverse events [
20].
This study was conducted to determine which packaging configuration of vaccines is the most appropriate to be used in a cold chain transport from pharmaceutical wholesalers to other healthcare clinics, hospitals and pharmacies. The findings of this study indicated that the size of storage box, packaging materials including materials used for cold box and insulator, and the number of coolant-packs, the arrangement of coolant-pack on top highly affects the cold retention, thus storage temperature and duration of intended temperature. With larger size of vaccine box, the number of coolant-packs required and the time to reach the designated storage temperature will be increased. In this study, configuration 1 was found to have the longest storage duration at desired temperature, 23 hours which are sufficient for transportation of both local and long-distance cold chain supply. The only drawback from this configuration is high possibility of freezing since the temperature went down to the lowest at 2.02℃ at first 2 hours. Additional layer of plastic wrap might be useful in order to prevent the temperature inside the vials from dropping below 2℃. Nonetheless, this measure needs to be further investigated. For transportation of small amount of vaccines at local districts, configuration 2, 4, and 6, respectively could serve as an alternative as the packaging are less bulky and lighter, thus easier for short transportation distance and time. On the other hand, the use of configuration 3 and 5 should be avoided since the internal temperature failed to fall below 8℃ at any time points.
As recent systematic review has indicated that 29 vaccine freezing cases reported in 45 included studies [
21]. The review suggested that a temperature fall below 2℃ is more common compared to the temperature rises above 8℃ during cold chain transportation. Several studies on effectiveness of cold chain supply of vaccines have been carried out globally [
21]. All of these studies concluded that the cold chain transport systems were not up to standard to ensure the quality and stability of the vaccine during transportation. Yauba et al. [
22] at Cameroon examined the vaccine exposure to temperatures outside the recommended range during storage and transport. Using experimental study design, 48 shipment boxes containing 10 vials of Diphtheria-Tetanus and Pertussis containing vaccines and a data logger were prepared and dispatched to various target health facilities throughout Cameroon [
22]. Of note, 83% of shipments were exposed to freezing (−0.5℃ to −23.8℃) at least once during their study. Meanwhile, a study conducted at regional and district vaccine stores and rural health facilities of India showed the temperatures in the boxes exceeded 8℃ ranged from 8.3% to 14.7% of their combined storage times and fell below 0℃ from 0.2% to 10.5% of these times. Up to 18% and 7% of the carrier boxes exposed to combined times in transit at <0 and >8℃, respectively [
17].
Meanwhile, in capital city of Kenya, Nairobi, a study on the influence of cold chain supply logistic on the safety of vaccines [
23]. The four influences being studied were the storage condition, packaging, transport system, and technical capacity. In the study on transport system, the research confirmed that the quality and conditions of transportation vans and carrier boxes were poor, along with the issue of improper documentation of actual temperature and timing of cold chain medicines throughout the transport process [
23].
Challenges in managing the cold chain in storage and during transportation could be overcome with a robust experimental study. Firstly, enhancement of good distribution practice requirements especially on management of cold chain products up to end users (clinics and private sector) is the necessary step to maintain quality, safety, and efficacy of products. The training also could increase awareness of new requirement and knowledge. Furthermore, vaccine lot release should also be implemented with proper documentation and review of lot summary protocol (LSP), along with cold chain inspection (CCI) comprising of physical checking and temperature monitoring. With satisfactory evaluation of LSP and CCI, then only vaccine lot release certificate will be issued by the drug regulatory agency.
The store personnel needs to be trained on Good Storage Practice especially the cold chain capacity requirements of a vaccine supply chain. Guidance on the number of vials, vaccine volumes and how to calculate cold chain storage needs for coolant-packs, the use of vaccine cold boxes, and the dry-storage capacity needed for immunization-related commodities should also be given [
2]. If the efficiency of the existing method is found to be low, attempt should be made to develop an effective supply chain method for the pharmaceutical wholesalers to deliver the vaccines to its customers without jeopardizing the cold chain. Overall, three core components of comprehensive vaccine cold chain management, as demonstrated in previous studies, are regular equipment maintenance, staff refresher course, and proper alert system connected to an auxiliary power [
24252627].
In conclusion, this study generated much needed evidence on duration and temperature maintenance of cold chain for the supply of quality vaccines and other biological products by pharmaceutical wholesalers. Polystyrene foam box could be effectively used if sealed with minimum five ice packs whereas large vaccine cold box with polyethylene interior lining and polypropylene insulation could be effectively used if sealed with minimum three ice pack. The configuration of packaging with coolant-pack, the size and material of the storage containers have a direct impact on the maintenance of cold chain temperature to ensure proper transportation of vaccines.
Go to :


