Abstract
Objectives
To analyze oncological outcomes according to the resection type and surgical margin following surgical treatment for primary spinal sarcoma.
Summary of Literature Review
Previous studies using registry databases have shown that surgery and negative margins were associated with improved survival for primary spinal sarcoma. However, few studies have comprehensively analyzed the clinical significance of the resection type and surgical margin for the oncological outcomes of this rare malignancy.
Materials and Methods
We retrospectively reviewed consecutive patients who underwent surgical resection for primary spinal sarcoma between 1997 and 2016 at two tertiary medical centers. Overall survival and the occurrence of local recurrence and distant metastasis were compared between the groups using Kaplan-Meier curve analysis and the log-rank test.
Results
Thirty-three patients (21 males, 12 females) with a mean age of 45.1 years and a median follow-up of 36 months were included. There were 13 (39.4%) chondrosarcomas, 12 (36.4%) osteosarcomas, and eight different histological diagnoses. The cohort was categorized into four groups: 1) total en bloc resection with a negative margin (n=12; 36.4%), 2) total en bloc resection with a positive margin: (n=5; 15.2%), 3) total piecemeal resection (n=12; 36.4%), and 4) subtotal resection (n=4; 12.1%). Total en bloc resection with a negative margin was associated with improved overall survival (p=0.030) and less distant metastasis (p=0.025) and local recurrence (p=0.004).
Go to : 
REFERENCES
1. Sundaresan N, Rosen G, Boriani S. Primary malignant tumors of the spine. Orthop Clin North Am. 2009 Jan; 40(1):21–36. v. DOI: 10.1016/j.ocl.2008.10.004.

2. Sohn S, Kim J, Chung CK, et al. A Nation-Wide Epide-miological Study of Newly Diagnosed Primary Spine Tumor in the Adult Korean Population, 2009-2011. J Korean Neurosurg Soc. 2017 Mar; 60(2):195–204. DOI: 10.3340/jkns.2016.0505.011.

3. Chi JH, Bydon A, Hsieh P, et al. Epidemiology and demo-graphics for primary vertebral tumors. Neurosurg Clin N Am. 2008 Jan; 19(1):1–4. DOI: 10.1016/j.nec.2007.10.005.

4. Kerr DL, Dial BL, Lazarides AL, et al. Epidemiologic and Survival Trends in Adult Primary Bone Tumors of the Spine. Spine J. 2019 Jul 12. pii: S1529-9430 (19)30846-0. DOI: 10.1016/j.spinee.2019.07. 003.

5. Mukherjee D, Chaichana KL, Parker SL, et al. Association of surgical resection and survival in patients with malignant primary osseous spinal neoplasms from the Surveillance, Epidemiology, and End Results (SEER) database. Eur Spine J. 2013; 22(6):1375–82. DOI: 10.1007/s00586-012-2621-4.

6. Arshi A, Sharim J, Park DY, et al. Chondrosarcoma of the Osseous Spine: An Analysis of Epidemiology, Patient Outcomes, and Prognostic Factors Using the SEER Registry From 1973 to 2012. Spine (Phila Pa 1976). 2017 May 1; 42(9):644–52. DOI: 10.1097/BRS.0000000000001870.
7. Arshi A, Sharim J, Park DY, et al. Prognostic determinants and treatment outcomes analysis of osteosarcoma and Ewing sarcoma of the spine. Spine J. 2017 May; 17(5):645–55. DOI: 10.1016/j.spinee.2016.11.002.

8. Schwab J, Gasbarrini A, Bandiera S, et al. Osteosarcoma of the mobile spine. Spine (Phila Pa 1976). 2012 Mar 15; 37(6):E381–6. DOI: 10.1097/BRS.0b013e31822fb1a7.

9. Schoenfeld AJ, Hornicek FJ, Pedlow FX, et al. Chondrosar-coma of the mobile spine: a review of 21 cases treated at a single center. Spine (Phila Pa 1976). 2012 Jan 15; 37(2):11926. DOI: 10.1097/BRS.0b013e31823d2143.

10. Hsieh PC, Xu R, Sciubba DM, et al. Long-term clinical outcomes following en bloc resections for sacral chordomas and chondrosarcomas: a series of twenty consecutive patients. Spine (Phila Pa 1976). 2009 Sep 15; 34(20):2233–9. DOI: 10.1097/BRS.0b013e3181b61b90.
11. Frankel HL, Hancock DO, Hyslop G, et al. The value of postural reduction in the initial management of closed injuries of the spine with paraplegia and tetraplegia. I. Paraplegia. 1969 Nov; 7(3):179–92. DOI: 10.1038/sc.1969.30.
12. Shah AA, Paulino Pereira NR, Pedlow FX, et al. Modi-fied En Bloc Spondylectomy for Tumors of the Thoracic and Lumbar Spine: Surgical Technique and Outcomes. J Bone Joint Surg Am. 2017 Sep 6; 99(17):1476–84. DOI: 10.2106/JBJS.17.00141.
13. Lim JB, Sharma H, MacDuff E, et al. Primary osteosarcoma of the spine: a review of 10 cases. Acta Orthop Belg. 2013 Aug; 79(4):457–62.
14. Dekutoski MB, Clarke MJ, Rose P, et al. Osteosarcoma of the spine: prognostic variables for local recurrence and overall survival, a multicenter ambispective study. J Neurosurg Spine. 2016 Jul; 25(1):59–68. DOI: 10.3171/2015.11. SPINE15870.

15. Liljenqvist U, Lerner T, Halm H, et al. En bloc spondylectomy in malignant tumors of the spine. Eur Spine J. 2008 Apr; 17(4):600–9. DOI: 10.1007/s00586-008-0599-8.

16. Fourney DR, Rhines LD, Hentschel SJ, et al. En bloc resection of primary sacral tumors: classification of surgical approaches and outcome. J Neurosurg Spine. 2005 Aug; 3(2):111–22. DOI: 10.3171/spi.2005.3.2.0111.

17. Talac R, Yaszemski MJ, Currier BL, et al. Relationship between surgical margins and local recurrence in sarcomas of the spine. Clin Orthop Relat Res. 2002 Apr; 397:127–32. DOI: 10.1097/00003086-200204000-00018.

Go to : 
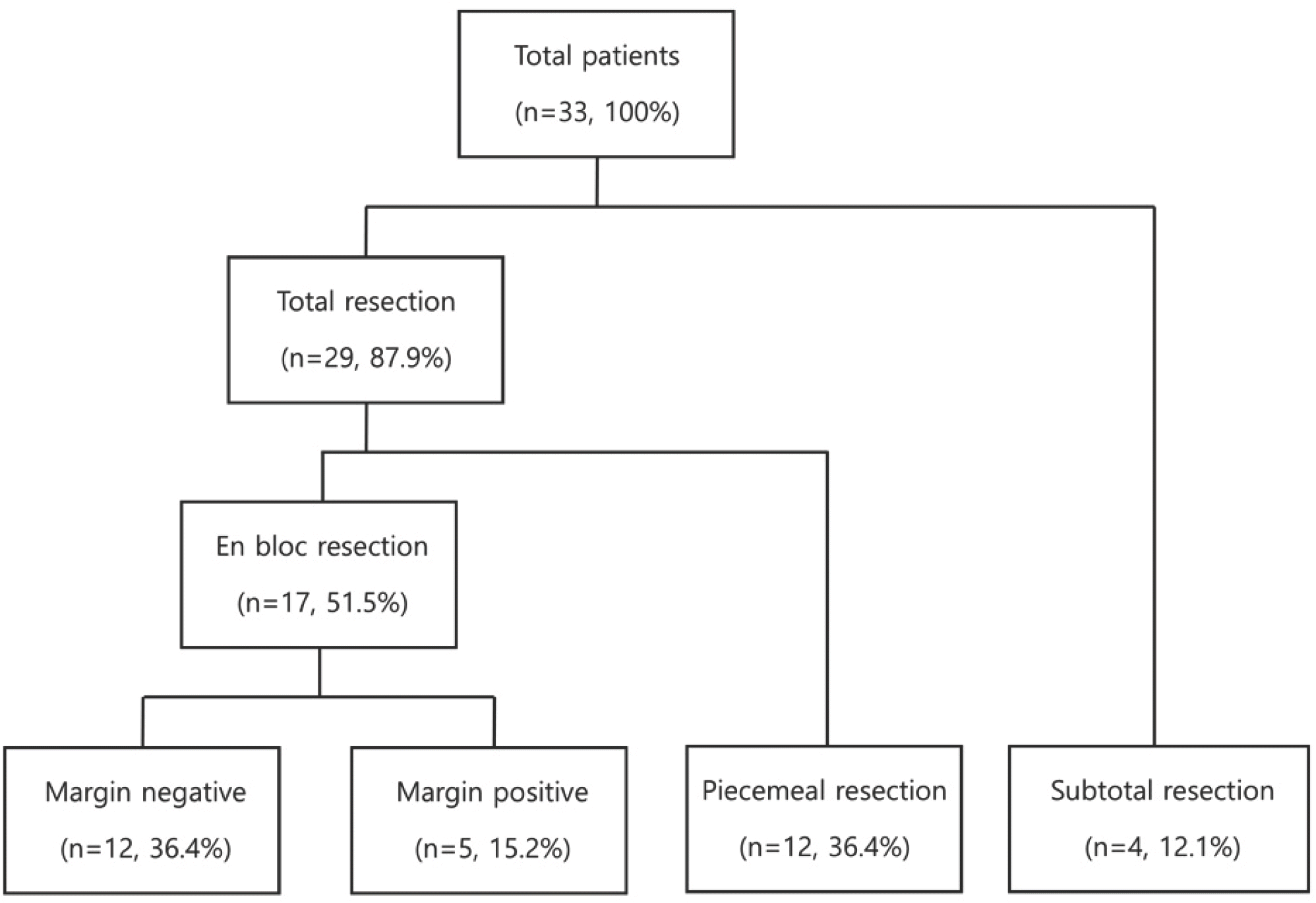 | Fig. 1.Distribution of patients according to the type of surgical resection and surgical margin. |
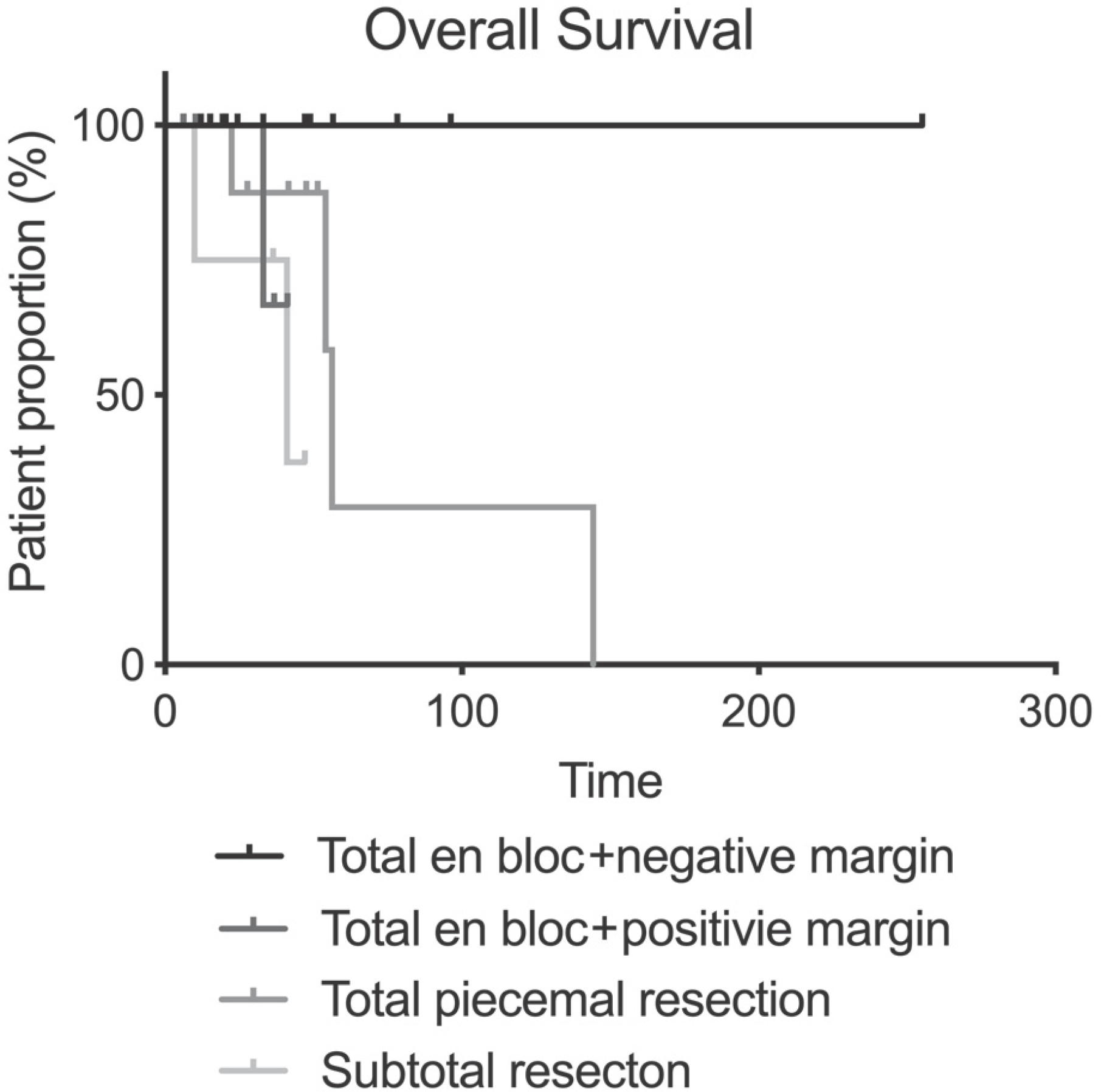 | Fig. 2.Kaplan-Meier survival curve of the total cohort showing an improvement in overall postoperative survival in patients who underwent total en bloc resection and had a negative margin |
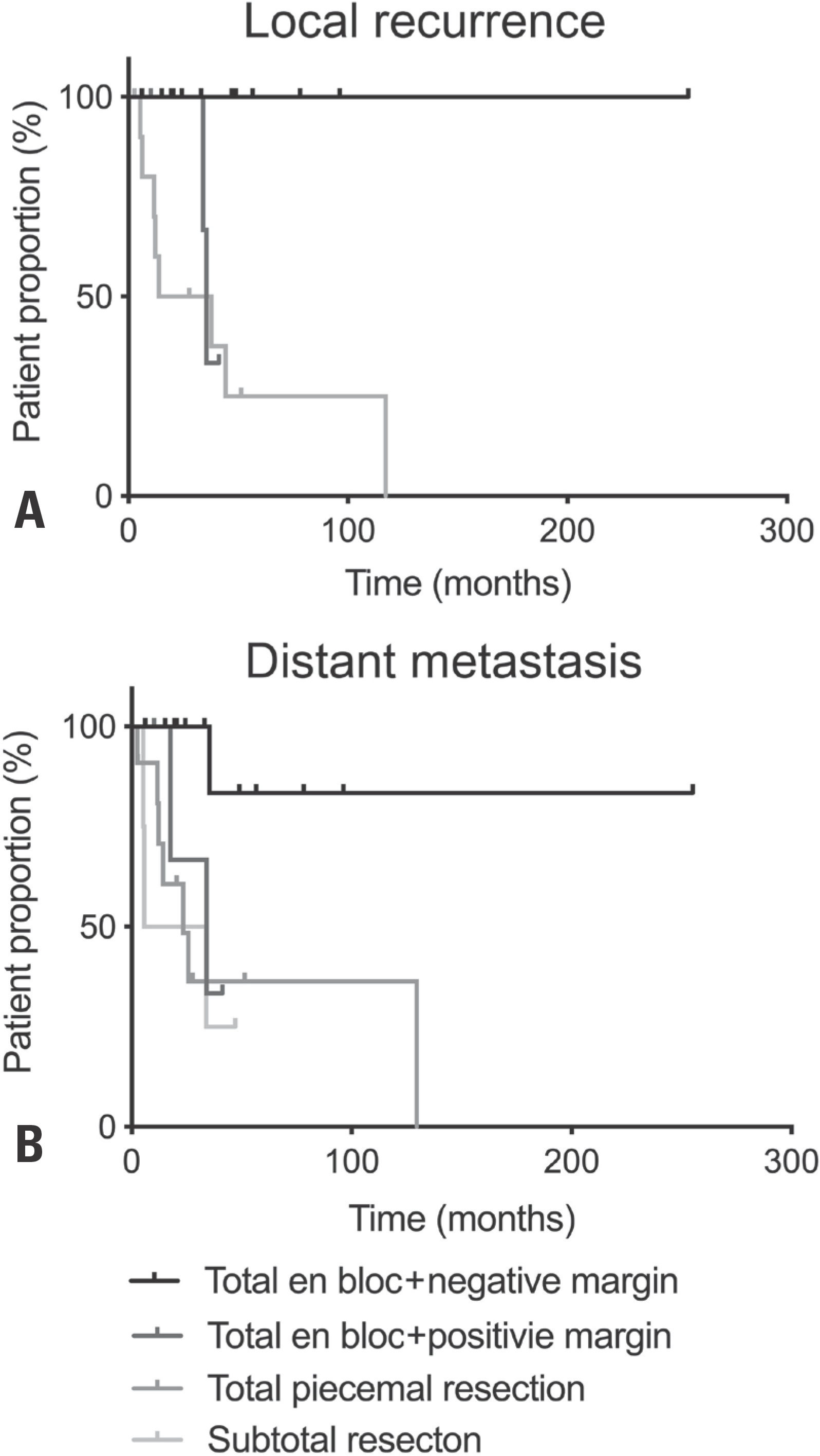 | Fig. 3.
(A) Kaplan-Meier survival curve for local recurrence showing significantly less recurrence in patients who underwent total en bloc re-section with a negative margin (p=0.004, log-rank test). (B) Kaplan-Meier survival curve for distant metastasis showing significantly less metastasis in patients who underwent total en bloc resection with a negative margin (p=0.025, log-rank test). |
 | Fig 4.
(A–D) A 63-year-old woman was diagnosed with osteosarcoma at the T7 level, which encased the descending thoracic aorta over the T5–8 levels. (E, F) The patient underwent total en bloc resection of the tumor, combined with replacement of the descending thoracic aorta by a thoracic surgeon. The postoperative X-ray is shown. (G, H) The specimen from the operation is shown. The patient experienced transient paraplegia due to spinal cord infarction, and currently ambulates using a walker. The patient had no evidence of disease after 4 years of follow-up. |
Table 1.
Summary of Osteosarcoma Patients
Table 2.
Summary of Chondrosarcoma Patients
| Case | Gender/Age | Site | Resection type | Surgical Margin | Local recurrence | Distant metastasis | Follow up (month) | Final status |
|---|---|---|---|---|---|---|---|---|
| 1 | F/41 | T7 | Total, En bloc | Negative | No | No | 255 | NED |
| 2 | F/63 | T10-11-12 | Total, En bloc | Negative | No | No | 20 | NED |
| 3 | M/32 | L2-3 | Total, En bloc | Negative | No | No | 25 | NED |
| 4 | F/60 | T4-8 | Total, En bloc | Negative | No | No | 24 | NED |
| 5 | F/55 | T9-10-11 | Total, En bloc | Positive | Yes | Yes | 36 | AWD |
| 6 | F/25 | L1 | Total, Piecemeal | No | No | 78 | NED | |
| 7 | M/76 | T11 | Total, Piecemeal | No | No | 51 | NED | |
| 8 | M/71 | T9-11 | Total, Piecemeal | No | No | 6 | NED | |
| 9 | M/48 | C4-5 | Total, Piecemeal | Yes | Yes | 47 | AWD | |
| 10 | M/36 | T4-6 | Total, Piecemeal | Yes | Yes | 41 | AWD | |
| 11 | M/43 | L4-5 | Total, Piecemeal | Yes | Yes∗ | 56 | DOD | |
| 12 | M/48 | T9-12 | Total, Piecemeal | Yes | Yes | 22 | DOD | |
| 13 | M/33 | C5 | Subtotal | NA | No | 47 | AWD |
Table 3.
Summary of Patients with Other Types of Sarcoma




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download