INTRODUCTION
Myeloid-derived suppressor cells (MDSCs) represent a heterogeneous population of immature myeloid cells (IMCs) consisting of precursors for granulocytes, macrophages, and dendritic cells (DCs) that are accumulated during chronic inflammation and tumor progression [
1]. IMCs in the bone marrow of healthy individuals quickly differentiate into mature granulocytes, macrophages, or DCs. MDSCs are of myeloid origin and are characterized by their immature state and remarkable ability to suppress T-cell responses [
2]. Given the key roles that they play in the immunosuppression network, the levels of MDSCs in cancer patients have been presumed to be of prognostic value [
3]. Previous studies have focused on the function and clinical relevance of MDSCs in gastrointestinal malignancies [
34]. However, studies regarding the clinicopathological impact of MDSCs on Korean patients with colorectal cancer (CRC) are limited. A systematic meta-analysis reported conflicting results on the relationship between MDSCs and prognostic factors of CRC such as mortality, TNM stage, metastasis, and lymph node involvement [
3]. Pretreatment peripheral immune status, including MDSCs and effector T-cells, was also reported as a predictive factor of the clinical outcome of patients with CRC [
5].
Moreover, lymphopenia is generally associated with an impaired cell-mediated immunity, while neutrophilia is a recognized response to systemic inflammation. Accordingly, the neutrophil-to-lymphocyte ratio (NLR), which is calculated as the neutrophil count divided by the lymphocyte count, is suggested as a marker for general immune responses to various stress stimuli [
6]. Although an elevated NLR in patients with CRC was found to predict poor clinical outcomes [
678910], findings on the direct impact of NLR on patient survival and the clinicopathological variables of the tumor remain inconclusive.
Therefore, this study investigated, retrospectively, the relationship between MDSCs, lymphocyte subsets, and NLR and the clinical features and prognoses of CRC patients.
MATERIALS AND METHODS
1. Patients
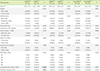
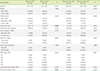
From 2014 to 2016, 68 patients with CRC were enrolled in this study. Peripheral blood samples were collected from the patients at the diagnosis stage. All patients with CRC received postoperative chemotherapy (fluorouracil and oxaliplatin), and three patients underwent additional postoperative radiotherapy. Clinical and laboratory data such as sex, tumor location, TNM staging, age, and preoperative carcinoembryonic antigen (CEA) were obtained from electronic medical records. The 68 patients with CRC were categorized into high and low groups for each cell population by a median split (%) of MDSCs, CD3+ total T-cells, CD4+ T-cells, CD8+ T-cells, CD19+ B-cells, and CD16+56+ natural killer cells (NK-cells). Additionally, these patients were categorized into high and low groups for NLR, which was calculated as the neutrophil count divided by the lymphocyte count obtained from electronic medical records.
2. MDSC and lymphocyte subset analysis
Flow cytometric analysis was performed on fresh peripheral blood for lymphocyte subsets and MDSCs at the diagnostic stage using a multicolor flow cytometry analyzer (BD FACSCalibur; BD Biosciences, San Jose, CA, USA). Lymphocyte subsets (T-cells, B-cells, and NK-cells) were analyzed using the following antibodies: IgG1 (Beckman Coulter, Inc., Brea, CA, USA), CD3/4 (Beckman Coulter, Inc.), CD3/8 (Beckman Coulter, Inc.), CD3/19 (Beckman Coulter, Inc.), CD3/16+56 (Beckman Coulter, Inc.), and CD45 PerCP (BD Biosciences, San Jose, CA, USA). Accordingly, the proportion of the CD3+ total T-cells, CD4+ T-cells, CD8+ T-cells, CD19+ B-cells, and CD16+56+ NK-cells was analyzed. Monocytic MDSCs (mMDSC) and granulocytic MDSCs (gMDSC) were analyzed using the following antibodies: mouse IgG2a allophycocyanin (APC) (BD Biosciences, Inc.), fluorescein isothiocyanate (FITC) mouse IgG1K isotype control (BD Biosciences, Inc.), phycoerythrin (PE)-mouse IgG1K isotype control (BD Biosciences, Inc.), CD11b-(D12) APC (BD Biosciences, Inc.), PE-mouse anti-human CD33 (BD Biosciences, Inc.), PE-cy7 mouse anti-human CD14 (BD Biosciences, Inc.), and FITC mouse anti-human CD15 Clone-W6D3 (BD Biosciences, Inc.). MDSC proportions were obtained using the following method: CD11b(+)/CD14(−)/CD33(+)/CD15(−) cells were regarded as mMDSCs and CD11b(+)/CD14(−)/CD33(+)/CD15(+) cells as gMDSCs.
3. Statistical Analysis
The χ2 test or Fisher's exact test was performed to determine the associations between MDSCs/lymphocyte subsets/NLR and other patient parameters, including sex, differentiation, tumor location, depth of invasion, lymph node involvement, TNM staging, lymphovascular invasion, perineural invasion, and recurrence. A Mann-Whitney U test was used to compare continuous variables such as age, tumor size, and preoperative CEA. Spearman's correlations were used to compare the relationship between lymphocyte subsets, NLR, and MDSC subsets. The proportion of MDSCs, lymphocyte subsets, and NLR according to TNM stage was analyzed using the Kruskal-Wallis test. The maximal χ2 method was employed to determine the cut-off point between the high NLR group and low NLR group. A Kaplan-Meier estimation was used to plot relapse-free survival (RFS) curves, and log-rank tests were employed to determine the difference between survival curves. Additionally, the Cox proportional hazards regression analysis was used to dissect the individual impact of prognostic factors for RFS. All tests were two tailed, and a P-value of less than 0.05 was considered statistically significant. All statistical analyses were performed using SPSS version 18.0 (SPSS Inc., Chicago, IL, USA) and R statistical software, version 2.13.0 (R Foundation for Statistical Computing).
4. Ethical statement
This study was approved by the Institutional Review Board of Chonnam National University Hwasun Hospital (IRB No. 2009-35) and performed in accordance with the principles of the Declaration of Helsinki. All samples were collected after obtaining written informed consent from the patients.
DISCUSSION
The functional interdependence between MDSCs, T-cells, and cancer cells in CRC pathogenesis has been previously reported, and indicates that elevated levels of MDSCs are correlated with advanced TNM stages and lymph node metastases [
11]. These researchers propose that CRC cells mediate MDSC induction, and these tumor-induced MDSCs can suppress T-cell proliferation and promote CRC cell growth. Zhang et al. [
12] also showed that increased MDSCs in patients with CRC are correlated with the cancer stage and metastases. They also suggested that a pharmacologic blockade of MDSCs should be considered in clinical trials. By contrast, Gabitass et al. [
13] reported that no significant association existed between cancer stages and MDSCs. Moreover, Sun et al. [
4] showed that patients with a high MDSC frequency did not have high odds for progressing into advanced stages, although their likelihood of metastases was significantly high.
In this study, no significant difference between lymph node metastases, TNM stage, tumor size, number of recurrence cases, and the histologic CRC types according to MDSC proportion was observed, except for the lymphovascular or perineural invasion, which was not observed in the high mMDSC group but was observed in the low mMDSC group. The previous studies by OuYang et al. [
11] and Zhang et al. [
12], unlike the current study on CRC patients with TNM stage II–III, were performed using a sufficient patient pool comprising cases of TNM stage I–IV, including distant metastases.
Previously, Tada et al. [
5] also suggested that a combined assessment of the immune-suppressive cells (mMDSC) and cytotoxic effector cells (CD4+ T-cells and CD8+ T-cells) might provide a more appropriate reflection of the patients' immune status and could yield a significant correlation between immune status and prognoses. Indeed, a short progression-free survival was observed in the high mMDSC group and in the low effector T-cell group in their report. However, in this study, we did not observe any prognostic impact of MDSCs or T-cells with respect to RFS. These conflicting results might have been caused by the heterogeneous nature of MDSCs themselves and the complex regulatory functions of different immune and non-immune cells with respect to tumors, as described previously [
14].
The prognostic value or role of NLR in patients with CRC has also been previously reported. For instance, Haram et al. [
9] reported that NLR is a useful biomarker in delineating patients with CRC who have a poor prognosis and may benefit from adjuvant therapies. In the study by Li et al. [
6], elevated pretreatment NLR predicted poor overall survival and progression-free survival in patients with CRC, and increased NLR was also significantly associated with poor tumor differentiation. Moreover, Kubo et al. [
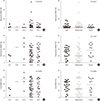
7] reported that not only the preoperative but also the postoperative NLR is a predictor of the long-term survival of patients with CRC. In this study, the high NLR group showed a slightly inferior RFS as compared with the low NLR group (
P=0.061), although the difference was not statistically significant (
Fig. 2). The overall survival analyses were not performed because all enrolled CRC patients were alive from the time of diagnosis to the time of retrospective analyses.
As mentioned above, we investigated the impact of MDSCs, lymphocyte subsets, and NLR for their prognostic value in CRC patients and demonstrated that the CD19+ B-cell proportion was the only independent prognostic factor for CRC relapse (HR=1.180; 95% CI: 1.040–1.337;
P=0.010). Additionally, an inferior RFS was found in the high CD19+ B-cell group as compared to the low CD19+ B-cell group after univariate Kaplan-Meier estimation (
Fig. 2). The high CD19+ B-cell group also showed bigger tumor sizes and more perineural invasions as compared to the low CD19+ B-cell group. According to a previous report [
15], B-cells inhibit anti-tumor T-cell responses through antigen non-specific mechanisms and migrate to the tumor site and induce the expression of immunosuppressive ligands or cytokines that contribute to the inhibition of the anti-tumor immune response. These explanations could be applied to the findings of the current study as well.
In the present study, the MDSC proportion did not serve as an important factor for predicting the clinical outcomes of patients with primary stage II and III CRC who underwent curative resection. This might be attributed to the high efficacy of surgical resection and postoperative chemotherapy in those patients. Alternatively, it might be due to the short duration before follow-up and an insufficient patient pool. Given these limitations, this study may be inadequate in evaluating the role of MDSC proportion in the regulation of patient immune function and in predicting the outcomes and clinicolaboratory parameters of postoperative patients with CRC. Moreover, our data were collected from a single center in Korea, so the findings may not be generalized to other institutions. Thus, the limited number of patients and the short follow-up period might preclude definitive conclusions on the clinical impact of MDSC, lymphocyte subset, and NLR for CRC patients.
In conclusion, the proportion of MDSCs in the peripheral blood of patients with CRC during the diagnostic stage was not associated with TNM stage or any clinicopathological parameters. In patients with operable stage II and III CRC, MDSCs were not a significant prognostic factor. However, among the lymphocyte subsets, the CD19+ B-cell proportion did show some ability to predict clinical outcomes. Thus, a preoperative evaluation of CD19+ B-cell proportion is recommended for predicting the clinical outcomes of patients with CRC. Further studies to accumulate more evidence are needed to determine the clinical impact of MDSCs, lymphocyte subsets, and NLR in patients with CRC.






 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download