Abstract
Background
Glycated hemoglobin (HbA1c) is a key biomarker for the monitoring of glycemic balance in patients with diabetes. The aim of this study was to evaluate the performance of a new system, the Tosoh HLC-723 G11 analyzer (Tosoh Corporation, Japan), compared to that of two routine diagnostic testing systems, Tosoh G8 (Tosoh Corporation) and Capillarys 2 Flex Piercing (Sebia, France).
Methods
Tosoh G11 was evaluated for precision, linearity, and carry-over, according to the Clinical and Laboratory Standard Institute's guidelines. Test results from clinical samples were compared between Tosoh G11 and the routine testing systems, Tosoh G8 and Capillarys 2 Flex Piercing.
Results
With respect to the precision of Tosoh G11, the test results for low- and high-concentration controls showed a coefficient of variation of less than 1.1%. Furthermore, the new device exhibited good linearity for HbA1c values ranging from 3.4% to 18.8%, and carry-over was not observed. HbA1c results for Tosoh G11 (N=143) correlated well with those for Tosoh G8 (r=0.9971) and Capillarys 2 Flex Piercing (r=0.9918).
Conclusions
Tosoh G11 demonstrated reliable analytical performance with good precision and linearity, and no carry-over results. In addition, its results were comparable to those of the existing instruments. Thus, the results of this evaluation suggest that Tosoh G11 is suitable for the routine diagnostic testing of HbA1c levels in clinical chemistry laboratories.
Figures and Tables
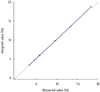 | Fig. 1Linearity of Tosoh HLC-723 G11 analyzer (Y=0.9768X+0.001182, R=0.999). The Solid line represents linear regression of the measured values and the dotted line is an equality line. |
 | Fig. 2Comparison of HbA1c values measured by the Tosoh HLC-723 G11 analyzer (tested instrument), Tosoh HLC-723 G8 analyzer (comparative instrument) and Capillarys 2 Flex Piercing (comparative instrument). A Passing and Bablok regression plot for Tosoh G11 and Sebia capillarys 2 (A) and for Tosoh G11 and Tosoh G8 (B). Bland- Altman difference plot for Tosoh G11 and Sebia capillarys 2 (C) and for Tosoh G11 and Tosoh G8 (D). |
References
1. Korean Centers for Disease Control and Prevention. Diabetes fact sheet in Korea 2012. http://www.diabetes.or.kr/temp/Diabetes_Fact_sheet2012.pdf.
2. Bonora E, Tuomilehto J. The pros and cons of diagnosing diabetes with A1C. Diabetes Care. 2011; 34 Suppl 2:S184–S190.

3. American Diabetes Association. Standards of medical care in diabetes--2010. Diabetes Care. 2010; 33 Suppl 1:S11–S61.
4. Wu X, Chao Y, Wan Z, Wang Y, Ma Y, Ke P, et al. A comparative evaluation of the analytical performances of Capillarys 2 Flex Piercing, Tosoh HLC-723 G8, Premier Hb9210, and Roche Cobas c501 Tina-quant Gen 2 analyzers for HbA1c determination. Biochem Med (Zagreb). 2016; 26:353–364.



5. NGSP. List of NGSP Certified Methods. Updated on Sep. 2019. http://www.ngsp.org/docs/methods.pdf.
6. Clinical and Laboratory Standards Institute. User verification of precision and estimation of bias; Approved guideline-Third edition. CLSI document EP15A3. Wayne, PA: Clinical and Laboratory Standards Institute;2014.
7. Clinical and Laboratory Standards Institute. Evaluation of the linearity of quantitative measurement procedures: A statistical approach; Approved guideline. CLSI document EP06-A. Wayne, PA: Clinical and Laboratory Standards Institute;2003.
8. Clinical and Laboratory Standards Institute. Measurement procedure comparison and bias estimation using patient samples: Approved guideline-Third edition. CLSI document EP09-A3. Wayne, PA: Clinical and Laboratory Standards Institute;2013.
9. QUALITY REQUIREMENTS. https://www.westgard.com/biodatabase1.htm#1.
10. Heinemann L, Freckmann G. Quality of HbA1c measurement in the practice: the German perspective. J Diabetes Sci Technol. 2015; 9:687–695.


11. Lee K, Kim SM, Jun SH, Song SH, Park KU, Song J. Evaluation of analytical performance of the D-100 hemoglobin testing system for hemoglobin A1c assay. J Lab Med Qual Assur. 2016; 38:95–101.

12. Woodworth A, Korpi-Steiner N, Miller JJ, Rao LV, Yundt-Pacheco J, Kuchipudi L, et al. Utilization of assay performance characteristics to estimate hemoglobin A1c result reliability. Clin Chem. 2014; 60:1073–1079.


13. Jaisson S, Leroy N, Meurice J, Guillard E, Gillery P. First evaluation of Capillarys 2 Flex Piercing® (Sebia) as a new analyzer for HbA1c assay by capillary electrophoresis. Clin Chem Lab Med. 2012; 50:1769–1775.


14. Jo Y, Lee Sy, Park Hi, Kim Y, Lee J, Kim Y, et al. Evaluation of HbA1c levels via the latex immunoturbidimetric method by using chemistry autoanalyzer. Lab Med Online. 2012; 2:10–14.





 PDF
PDF ePub
ePub Citation
Citation Print
Print






 XML Download
XML Download