Abstract
Background
Serum α-fetoprotein (AFP) test is useful for the diagnosis of hepatocellular carcinoma (HCC) and monitoring its recurrence after treatment. In Korea, patients with a higher risk of HCC are tested every six months for AFP, as a screening process to detect HCC. We aimed to assess the analytical performance of the AFP test in the HISCL-5000 (Sysmex Corporation, Japan) instrument, which has been released recently.
Methods
HISCL-5000 AFP assay was evaluated for precision, linearity, and comparison, according to the CLSI (Clinical and Laboratory Standards Institute) guidelines. For precision assessment, two control materials and one pooled human serum were measured twice a day for 20 days in duplicate. For linearity, five levels of material were produced covering the upper and lower limits of the measurable range and each of these was measured four times. Method comparison was conducted between HISCL-5000 and ADVIA Centaur XP (Siemens, Germany) with 500 samples consisting of 315 HCC, 57 benign liver disease, and 128 healthy subjects.
Results
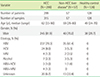
Repeatability was between 2.44% and 9.19%, and within-laboratory precision was between 6.47% and 9.53%. Coefficient of determination (R2) was 0.9975, with the range of 1.2-1824.3 ng/mL. Correlation coefficient (r) was 0.9862 between HISCL-5000 and Centaur XP. Overall percent agreement was 95.2% and difference percentage between the two instruments was 7.0%. Reference interval from healthy subjects was 1.30-11.42 ng/mL.
Figures and Tables
 | Fig. 1Method comparison between HISCL-5000 and Centaur XP analyzers for the measurement of serum AFP. (A) Regression line with Weighted Ordinary Linear Regression. (B) Bland-Altman Difference plot. |
 | Fig. 2Serum AFP levels in different patient groups. Gray points refer to each value, horizontal lines below and above refer to the 25th and 75th percentile values, and horizontal lines within boxes indicate median levels. |
References
1. Ministry of Health and Welfare, National Cancer Center. Quality guidelines of liver cancer screening, 2nd rev. 2006. http://www.ncc.re.kr 2016.
2. Bialecki ES, Di Bisceglie AM. Diagnosis of hepatocellular carcinoma. HPB (Oxford). 2005; 7:26–34.



3. Korean Liver Cancer Study Group (KLCSG). National Cancer Center, Korea (NCC). 2014 Korean Liver Cancer Study Group-National Cancer Center Korea practice guideline for the management of hepatocellular carcinoma. Korean J Radiol. 2015; 16:465–522.


4. Ko YS, Bae JH, Sinn DH, Gwak GY, Kang W, Paik YH, et al. The clinical significance of serum alpha-fetoprotein in diagnosing hepatocellular carcinoma in a health screening population. Korean J Gastroenterol. 2017; 69:232–238.


5. Mitsuhashi N, Kobayashi S, Doki T, Kimura F, Shimizu H, Yoshidome H, et al. Clinical significance of alpha-fetoprotein: involvement in proliferation, angiogenesis, and apoptosis of hepatocellular carcinoma. J Gastroenterol Hepatol. 2008; 23:e189–e197.
6. Yang X, Zhang Y, Zhang L, Zhang L, Mao J. Silencing alpha-fetoprotein expression induces growth arrest and apoptosis in human hepatocellular cancer cell. Cancer Lett. 2008; 271:281–293.


7. Tangkijvanich P, Anukulkarnkusol N, Suwangool P, Lertmaharit S, Hanvivatvong O, Kullavanijaya P, et al. Clinical characteristics and prognosis of hepatocellular carcinoma: analysis based on serum alpha-fetoprotein levels. J Clin Gastroenterol. 2000; 31:302–308.

8. Yamashita T, Ji J, Budhu A, Forgues M, Yang W, Wang HY, et al. EpCAM-positive hepatocellular carcinoma cells are tumor-initiating cells with stem/progenitor cell features. Gastroenterology. 2009; 136:1012–1024.


9. Clinical and Laboratory Standards Institute. Evaluation of precision of quantitative measurement procedures; Approved guideline—Third edition. CLSI document EP05-A3. Wayne, PA: Clinical and Laboratory Standards Institute;2014.
10. Clinical and Laboratory Standards Institute. Evaluation of the linearity of quantitative measurement procedures: a statistical approach; Approved guideline. CLSI document EP06-A. Wayne, PA: Clinical and Laboratory Standards Institute;2003.
11. Clinical and Laboratory Standards Institute. Measurement procedure comparison and bias estimation using patient samples; Approved gui-deline—Third edition. CLSI document EP09-A3. Wayne, PA: Clinical and Laboratory Standards Institute;2013.
12. Clinical and Laboratory Standards Institute. Defining, establishing, and verifying reference intervals in the clinical laboratory; Approved standard—Third edition. CLSI document C28-A3. Wayne, PA: Clinical and Laboratory Standards Institute;2010.
13. Park Y, Park Y, Park J, Kim HS. Evaluation of the UniCel™ DxI 800 immunoassay analyzer in measuring five tumor markers. Yonsei Med J. 2012; 53:557–564.

14. Kim HY, Lee SY, Park HD. Performance evaluation of the SelexOn analyser for seven biomarkers. J Lab Med Qual Assur. 2014; 36:30–38.





 PDF
PDF ePub
ePub Citation
Citation Print
Print






 XML Download
XML Download