Abstract
Splenic B-cell lymphomas (SBCLs) show characteristically pronounced splenomegaly without significant lymphadenopathy. Distinguishing hairy cell leukemia (HCL) from other SBCLs (splenic marginal zone lymphoma [SMZL], variant HCL [v-HCL], and splenic diffuse red pulp small B-cell lymphoma [SDRPL]) is essential to determine suitable treatments and prognoses. With advances in diagnostic modalities and therapies, splenectomy is not commonly performed, and thus diagnosis of HCL must be based on the results obtained using blood and bone marrow samples. Annexin A1 is known as the most specific marker for HCL. There has yet been no report of the assessment of annexin A1 immunostaining from Korea. In this study we analyzed samples from 13 Korean patients with SBCLs (three HCL, three v-HCL, six SMZL, and one SDRPL) from May 2001 to December 2016. Immunohistochemical analyses for annexin A1 and CD20 were performed using bone marrow sections; molecular analyses for detection of the BRAF V600E mutation were also performed. All HCL patients showed positive results for annexin A1 immunostaining and the presence of the BRAF V600E mutation, and negative results for other SBCLs. Our results confirmed the high specificity of annexin A1 and the BRAF V600E mutation as HCL markers. Molecular analysis requires expensive equipment and substantial manpower. Annexin A1 is a better alternative as an HCL marker than the BRAF V600E mutation in terms of cost-effectiveness.
Splenic B-cell lymphomas (SBCLs) that characteristically exhibit pronounced splenomegaly without significant lymphadenopathy include; splenic marginal zone lymphoma (SMZL), hairy cell leukemia (HCL), and unclassifiable SBCLs (including the provisional clinical entities specified by the World Health Organization; variant HCL [v-HCL] and splenic diffuse red pulp small B-cell lymphoma [SDRPL]) [1]. Distinguishing HCL among the subtypes of SBCLs is crucial because HCL is uniquely sensitive to nucleosides (purine analogs) [2]. Other clinical entities that mimic this disease, such as v-HCL and SMZL do not respond to HCL therapies and show a significantly lower survival rate [1]. Diagnosis of HCL has relied on histopathologic examination of the spleen. However, with advances in diagnostic modalities and therapies, splenectomy is not commonly performed, and diagnosis must be based on findings from studies using blood and bone marrow samples. Distinguishing HCL from other SBCLs requires the use of several methods, including immunophenotyping, immunohistochemistry, and molecular genetic studies, along with histopathologic examination of tissue samples. Immunophenotypic analysis using multi-parameter flow cytometry (FCM) is essential to establish differential diagnosis of SBCLs. FCM results revealed that, CD20, CD22, CD11c, CD25, and CD103 are expressed in HCL [34]. The immunophenotype of v-HCL has been reported to be typically positive for CD20, CD22, CD11c, CD103, and negative for CD25 [1345]. SMZL cells collected from patients usually show a non-specific immunophenotype (negative for CD5, CD10, CD23 or CD103). Unlike most cases of SMZL, SDRPL is similar to v-HCL. SDRPL cells are less likely to express CD103 than v-HCL [678].
Specific expression of annexin A1 in HCL was initially discovered using gene expression profiling [9]. Annexin A1 has been suggested to play an important role in inflammatory response, cell proliferation, cell signaling, phagocytosis, and carcinogenesis [10]. Annexin A1 is known as the most specific marker for HCL, as it was not found to be expressed in SBCLs other than HCL in an immunohistochemical study [11]. As healthy B cells do not express the protein, aberrant expression of annexin A1 is probably the result of neoplastic transformation of B cells, most likely of memory-type B cells [11]. Although HCL cases have often been reported [1213], there has been no official report of assessment of annexin A1 immunostaining from Korea. Thus, we have performed immunohistochemical analyses in order to evaluate the diagnostic utility of annexin A1, along with immunophenotyping and molecular studies for detection of the BRAF V600E mutation.
A total of 13 Korean patients (three HCL, three v-HCL, six SMZL, and one SDRPL) were retrospectively enrolled and their clinical characteristics, complete blood counts (CBC), peripheral blood, bone marrow morphology, immunophenotypes and molecular studies for detection of BRAF V600E (gain-of-function mutations of BRAF [v-raf murine sarcoma viral oncogene homolog B1] serine/threonine protein kinase) were analyzed between May 2001 to December 2016. Each patient had been previously diagnosed on the basis of clinical information, histomorphology and immunophenotypic characteristics in accordance with the 2017 World Health Organization classification of tumors of hematopoietic and lymphoid tissues [1].
Immunohistochemical studies, using CD20 (DAKO, Glostrup, Denmark) and annexin A1 (Cell Marque, Rocklin, CA, USA) immunostaining were performed using formalin-fixed and paraffin-embedded bone marrow core biopsy sections using an automated immunostainer (Ventana Benchmark XT staining system, Ventana Medical Systems, Tucson, AZ, USA) according to the manufacturer's protocols. Hematoxylin and eosin staining and CD20 immunostaining were performed to determine the involvement of lymphoma. Annexin A1 immunostaining in CD20-positive malignant cells was also evaluated. Immunophenotyping was performed using flow cytometry using the dual-laser FACSCanto™ II flow cytometer (Becton-Dickinson, San Jose, CA, USA) with a panel of lymphoid cell-associated monoclonal antibodies against CD5, CD10, CD19, CD20, CD22, CD25, CD38, CD103, CD11c, and surface immunoglobulin (Becton-Dickinson). Data were acquired and analyzed using the BD FACSDiva™ software (Becton-Dickinson).
Genomic DNA was isolated from bone marrow specimens with written informed consent from patients. Real-time PCR was performed using Real Q BRAF V600E detection kits (BioSewoom Inc., Seoul, Korea) and the 7500 Fast Real-Time System (Applied Biosystems, Foster City, CA, USA) as previously described [14]. Mutant enrichment 3′-modified oligonucleotide (MEMO)-PCR and sequencing analysis for BRAF V600E mutation were performed as previously described [15].
Basic characteristics and findings of experiments from samples of patients are summarized in Table 1. All HCL patients had anemia and thrombocytopenia and all v-HCL patients had leukocytosis as seen upon performing automated blood counts. Bone marrows of all HCL patients were packed with neoplastic cells (3/3, 100%). Bone marrow findings from 40% (4/10) of the other SBCL samples showed a nodal infiltration pattern, and 20% samples (2/10) showed an intrasinusoidal infiltration pattern. All patient samples were negative for CD5 and CD10, and positive for CD19 and CD20 expression. All HCL cells collected from patients showed expression of the following antigens: CD25 (3/3, 100%), CD11c (2/2, 100%), and CD103 (3/3, 100%). All v-HCL patient samples were positive for CD11c (3/3, 100%) and CD103 (3/3, 100%) expression, however, they were negative for CD25 expression (0/3, 0%). Thirty-three percent (1/3) of the SMZL patient samples were CD25(+), and all SMZL samples were CD11c(−), and CD103(−). One sample from a SDRPL patient showed a CD11c(+)/CD103(partial)/CD25(−) phenotype. Chromosomal analyses revealed that three SMZL samples had abnormal karyotype: patient 3, 47,XX,t(2;7)(p13;p13),+12,t(14;16)(q32;p13.1),t(18;19)(q21.1;q13.1)[15]/46,XX[11]; patient 4, 46,XY,del(5)(q13q22)[3]/46,XY[17]; patient 5, 46,XY,dup(1)(q21q32),add(5)(q35),add(6)(q22),i(6)(p10),del(7)(q32)[15]/47,sl, +i(6)(p10)[3]/46,XY[2]. All patients, excluding the 3 SMZL cases described above had normal chromosomes. All HCL patient samples (3/3, 100%) were positive for annexin A1 immunohistochemical staining (Fig. 1) and negative for other SBCL immunostaining (0/10, 0%). Rates for positive annexin A1 expression ranged from about 20 to 100 percent. All HCL patient samples (3/3, 100%) were positive for the BRAF V600E mutation as found using real time PCR, MEMO-PCR and sequencing. In contrast, other SBCL patient samples were negative for the BRAF V600E mutation (0/7, 0%).
Morphologic features observed upon bone marrow examination may not be sufficient to distinguish between HCL and the other subtypes. However, a few marrow patterns are helpful in establishing differential diagnosis of SBCLs. SMZL shows various patterns of bone marrow infiltration, typically with nodular, interstitial, and intrasinusoidal distribution [6]. Specifically, in SMZL patients, the nodular pattern excludes hairy cell leukemia as observed upon performing bone marrow biopsy [1]. Bone marrow results that help in diagnosing HCL show interstitial or diffuse infiltration of lymphocytes. V-HCL may tend to exhibit less extensive marrow involvement than HCL and can show interstitial, predominantly sinusoidal, or diffuse patterns of marrow infiltration [516]. In our study (Table 1), nodular patterns were observed only in bone marrows of patients with SMZL. Bone marrows from SBCL patients showed higher proportions of diffuse or interstitial patterns than those from SMZL patients. Moreover, HCL tissue samples exhibited more extensive marrow involvement than v-HCL. In this report, immunophenotypic analysis was helpful in classifying samples as those of HCL, v-HCL, and SDRPL. However, immunophenotypic analysis has some limitations: a fresh sample of tissue is required, appropriate cell surface markers must be selected, and samples must be analyzed by an expert.
We observed positive annexin A1 immunostaining in samples from all HCL patients and negative in the other SBCL samples. Annexin A1 shows high specificity for HCL and thus is useful for accurate diagnosis. BRAF V600E, like annexin A1, is also a highly-specific marker for HCL, however, Sanger sequencing for BRAF V600E requires longer duration and is expensive than immunostaining for annexin A1. Besides, not all HCL patients show BRAF V600E mutations. One report suggested that 11 of 53 (21%) patients with HCL may not have BRAF mutations [17]. Of these 11 HCL patients, seven had bone marrow biopsies which provided adequate amounts of tissue samples to stain for annexin A1, and all were positive for annexin A1 [17]. However, annexin A1 is strongly expressed in healthy myeloid cells which makes it difficult to get accurate results. Besides, annexin A1 does not stain all malignant cells of HCL. Although more than 90% of bone marrow biopsies from HCL patients were positive for CD20, annexin A1 results varied from 20% to 90% (Table 1). To overcome such shortcomings, annexin A1 is used for diagnosis of HCL with extensive marrow involvement or with extramedullary disease, and CD20 staining must be performed simultaneously to distinguish HCL cells from nonlymphoid elements in the marrow.
In order to diagnose HCL, morphologic and immunophenotypic features of patients, positive annexin A1 immunostaining and/or the presence of the BRAF V600E mutation are helpful. Of these, immunostaining is simple and can be analyzed rapidly. In the near future, advanced technology might be used to diagnose hematologic malignancies [18]. However, expensive equipment and considerable manpower will be required for such methods. For these reasons, immunostaining for markers like annexin A1 is useful and economical.
In conclusion, our results support high specificity of annexin A1 and BRAF V600E mutations as markers for diagnosis of HCL, as well as the diagnostic value of standard HCL markers such as CD11c, CD25, and CD103. Annexin A1 immunostaining is thus a better alternative than detection of BRAF V600E mutations or performing immunophenotypic analyses.
Figures and Tables
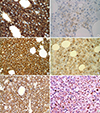 | Fig. 1Three cases of classical hairy cell leukemia. CD20 (A, 1,000×), and annexin A1 (B, 1,000×) immunostaining for tissue samples from patient 7. CD20 (C, 400×), and annexin A1 (D, 400×) immunostaining for tissue samples from patient 8. CD20 (E, 400×), and annexin A1 (F, 400×) immunostaining for tissue samples from patient 9. |
Table 1
Basic characteristics and results of annexin A1 immunostaining with immunophenotypes and BRAF V600E

*Previously reported by Shin et al. [14].
Abbreviations: SMZL, splenic B-cell marginal zone lymphoma; HCL, hairy cell leukemia; v-HCL, variant hairy cell leukemia; SDRPL, splenic diffuse red pulp small B-cell lymphoma; BM, bone marrow; Pos, positive; Neg, negative; NA, not available.
References
1. Swerdlow SH, Campo E, editors. WHO classification of tumours of haematopoietic and lymphoid tissues. Revised 4th ed. Lyon, France: World Health Organization;2017.
3. Dong HY, Weisberger J, Liu Z, Tugulea S. Immunophenotypic analysis of CD103+ B-lymphoproliferative disorders: hairy cell leukemia and its mimics. Am J Clin Pathol. 2009; 131:586–595.

4. Venkataraman G, Aguhar C, Kreitman RJ, Yuan CM, Stetler-Stevenson M. Characteristic CD103 and CD123 expression pattern defines hairy cell leukemia: usefulness of CD123 and CD103 in the diagnosis of mature B-cell lymphoproliferative disorders. Am J Clin Pathol. 2011; 136:625–630.

5. Matutes E, Wotherspoon A, Catovsky D. The variant form of hairy-cell leukaemia. Best Pract Res Clin Haematol. 2003; 16:41–56.

6. Ponzoni M, Kanellis G, Pouliou E, Baliakas P, Scarfò L, Ferreri AJ, et al. Bone marrow histopathology in the diagnostic evaluation of splenic marginal-zone and splenic diffuse red pulp small B-cell lymphoma: a reliable substitute for spleen histopathology? Am J Surg Pathol. 2012; 36:1609–1618.

7. Kanellis G, Mollejo M, Montes-Moreno S, Rodriguez-Pinilla SM, Cigudosa JC, Algara P, et al. Splenic diffuse red pulp small B-cell lymphoma: revision of a series of cases reveals characteristic clinico-pathological features. Haematologica. 2010; 95:1122–1129.

8. Traverse-Glehen A, Baseggio L, Bauchu EC, Morel D, Gazzo S, Ffrench M, et al. Splenic red pulp lymphoma with numerous basophilic villous lymphocytes: a distinct clinicopathologic and molecular entity? Blood. 2008; 111:2253–2260.

9. Basso K, Liso A, Tiacci E, Benedetti R, Pulsoni A, Foa R, et al. Gene expression profiling of hairy cell leukemia reveals a phenotype related to memory B cells with altered expression of chemokine and adhesion receptors. J Exp Med. 2004; 199:59–68.

10. Zhu DW, Liu Y, Yang X, Yang CZ, Ma J, Qiao JK, et al. Low Annexin A1 expression predicts benefit from induction chemotherapy in oral cancer patients with moderate or poor pathologic differentiation grade. BMC Cancer. 2013; 13:301.

11. Falini B, Tiacci E, Liso A, Basso K, Sabattini E, Pacini R, et al. Simple diagnostic assay for hairy cell leukaemia by immunocytochemical detection of annexin A1 (ANXA1). Lancet. 2004; 363:1869–1870.

12. Jang MJ, Rhyu KH, Chong SY, Oh D, Kang MS. A case of hairy cell leukemia, variant. Korean J Hematol. 2004; 39:167–171.
13. Kang SB, Park WS, Lee KM, Lee DS, Kim KW, Kim HJ, et al. A case of hairy cell leukemia. Korean J Hematol. 2000; 35:92–96.
14. Shin SY, Lee ST, Kim HJ, Ki CS, Jung CW, Kim JW, et al. BRAF V600E and MAP2K1 mutations in hairy cell leukemia and splenic marginal zone lymphoma cases. Ann Lab Med. 2015; 35:257–259.

15. Lee ST, Kim JY, Kown MJ, Kim SW, Chung JH, Ahn MJ, et al. Mutant enrichment with 3′-modified oligonucleotides a practical PCR method for detecting trace mutant DNAs. J Mol Diagn. 2011; 13:657–668.
16. Shao H, Calvo KR, Grönborg M, Tembhare PR, Kreitman RJ, Stetler-Stevenson M, et al. Distinguishing hairy cell leukemia variant from hairy cell leukemia: development and validation of diagnostic criteria. Leuk Res. 2013; 37:401–409.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download