This article has been
cited by other articles in ScienceCentral.
Abstract
We report a rare case of hyperglycemia-related osmotic demyelination syndrome (ODS) with focus on the imaging findings. A 61-year-old man with diabetes was admitted for general weakness and severe thirst. A few days later, he complained of dysarthria, dysphasia, and dysmetria. Laboratory examinations showed significant hyperglycemia, but normal electrolyte levels. Brain MRI revealed T2-signal abnormalities that were symmetrical, non-space occupying, and located in the central pons with a peripheral sparing pattern, which were suggestive of ODS. In addition, subsequent MRI revealed progression of signal hyperintensity; however, the patient's symptoms improved.
초록
우리는 이전까지 드물게 보고된 고혈당 관련 삼투압성 탈수초화 증후군의 연속 자기공명영상 소견을 보고하고자 한다. 당뇨병을 앓고 있는 61세 남자 환자가 전신쇠약과 심한갈증을 주소로 내원하였고 며칠 후 구음장애, 삼킴장애, 운동조절장애를 보였다. 혈액검사상 고혈당을 제외한 전해질 수치는 정상이었다. 뇌 자기공명영상에는 교뇌에서 대칭적이고 종괴 효과가 없으며 주변 부위를 침범하지 않는 T2 신호 이상이 보여 삼투압성 탈수초화 증후군에 합당한 소견이었다. 환자는 임상적인 증상의 호전을 보였으나 추후 촬영한 뇌 자기공명영상은 악화되는 소견을 보였다.
Keywords: Demyelinating Diseases, Hyperglycemia, Magnetic Resonance Imaging
INTRODUCTION
Osmotic demyelination syndrome (ODS) is a non-inflammatory demyelinating disease that occurs in a variety of clinical settings, but most commonly in association with a rapid rise in plasma osmolality during correction of chronic hyponatremia (
1). It has recently been reported that ODS can also occur in the context of a rapid increase serum osmolality in the absence of rapid changes in serum sodium levels (
2).
In this article, we report a rare presentation of ODS that occurred secondary to a hyperglycemic hyperosmolar state (HHS) with normal corrected serum sodium levels.
CASE REPORT
A 61-year-old man presented to the emergency department due to general weakness and severe thirst. He had a medical history significant for type II diabetes mellitus, hypertension, dementia, and liver cirrhosis. His compliance to medication was poor, especially prescribed insulin. The initial suspicion was HHS; therefore, he was admitted to correct hyperglycemia. In the following few days of hospitalization, he complained of progressive motor weakness and could not stand alone. Dysarthria, dysphasia, and dysmetria, which progressively worsened over the previous 10 days, also emerged despite adequate control of blood sugar levels.
On admission, significant hyperglycemia [33.5 mmol/L (627 mg/dL)] was noted, and glycated hemoglobin level was 18.1%. Other investigations revealed a sodium level of 133 mEq/L, potassium 3.8 mEq/L, blood urea nitrogen 43.9 mg/dL, with a calculated serum osmolality of 324 mOsm/kg.
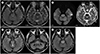
MRI was performed 8 days after admission; at that time, his neurological symptoms appeared. Prominent high signal intensity was observed within the pons on subsequent fluid-attenuated inversion recovery (FLAIR) and T2-weighted imaging (
Fig. 1A). These signal abnormalities were symmetrical, non-space occupying, and located in the central pons with peripheral sparing pattern, which are findings suggestive of ODS. Diffusion-weighted imaging (DWI) revealed subtle increased signal intensity in the pons without definite low apparent diffusion coefficient values, findings consistent with T2-shine-through (
Fig. 1B). The basal ganglia, thalami, and both cerebral hemispheres were within normal limits. No significant abnormalities were noted on magnetic resonance angiography.
Follow-up MRI performed 10 days later revealed a newly developed lesion in the posterior tegmentum portion of the upper pons and bilateral brachium pontis (
Fig. 1C). Additional follow-up MRI performed 1 month later revealed no newly developed lesion; however, overall signal hyperintensity in the affected area was more conspicuous and confluent, suggesting lesion progression (
Fig. 1D).
The final diagnosis was ODS secondary to hyperosmolar hyperglycemia. Uncontrolled diabetes mellitus was managed primarily by hydration and insulin infusion, with close blood glucose monitoring. Other conservative therapies and physical rehabilitation were also implemented. Under comprehensive therapy, the patient's general condition and neurological symptoms improved. He slowly regained mobility over the course of several weeks. His swallowing, speech, and eye movement gradually recovered.
DISCUSSION
Central pontine myelinolysis (CPM) was first described in 1959 in a patient who exhibited distinctive myelin loss in the pons, which was attributed to alcoholism or malnutrition (
3). This type of myelinolysis can affect areas outside the pons, a condition that is referred to as extrapontine myelinolysis. Basal ganglia and cerebral white matter and, less commonly, the peripheral cortex, hippocampi, and lateral geniculate bodies, were sites of extrapontine myelinosis. The phrase “osmotic demyelination syndrome” is preferred to account for both entities (
4).
It is widely accepted that the most common cause of ODS is hyperosmotic stress resulting from rapid correction of severe hyponatremia (
567). Other conditions related to ODS include alcoholism, malnutrition, prolonged diuretic use, burns (in the context of hypernatremia), postliver transplant, AIDS, folate deficiency, and hypoglycemia (
689). The association of ODS secondary to HHS has seldom been reported.
MRI findings in our patient revealed high signal intensity in the central pons on T2-weighted FLAIR and DWI, which was suggestive of ODS. Differential diagnosis included tumorous condition, and other demyelinating diseases including multiple sclerosis.
The patient underwent MRI for evaluation of headache one month before admission, which confirmed unremarkable findings in the pons (data not shown). In this acutely developed pontine lesion, tumorous conditions were ruled out. Multiple sclerosis was excluded because there was no other signal abnormality at the site such as Dawson's finger (T2 high signal intensity propagating centrifugally along the medullary venules and arranged perpendicular to the lateral ventricles), which is a typical finding in multiple sclerosis (
10).
Symmetrical high signal intensity with peripheral sparing is a characteristic finding of ODS on T2-weighted and FLAIR MRI. It is also called the “trident-shape” sign, resulting from sparing of the ventrolateral pons and the pontine portion of the corticospinal tracts. Low signal intensity throughout the affected areas on T1-weighted imaging and non-enhancement after the administration of contrast material are the classic findings, and do not have a mass effect (
10).
A newly developed lesion in the posterior tegmentum portion on follow-up MRI involved the medial longitudinal fasciculus, which correlated with our patient's symptom of lateral gaze limitation. A bilateral brachium pontis lesion usually reflects the involvement of the pontocerebellar fiber; however, gait ataxia could not be confirmed because of the patient's inability to stand due to motor weakness.
The present case is special in several respects. First, ODS occurred only in conditions of hyperosmolality. In the absence of any proven hyponatremia, chronic hyperglycemia was the likely cause for CPM in this patient. However, liver cirrhosis may accelerate ODS because of limitations in the capacity of the liver for glucose disposal, and impairment of water shifting balance. Second, a clinical/radiological discrepancy was noted. Imaging findings of ODS reveal gradual progression on follow-up studies, despite the prompt correction of blood sugar levels and improvement of neurological symptoms.
We report a rare case of hyperglycemia-related ODS with a focus on the imaging findings and it is important that in patient with diabetes mellitus, hyperglycemia is one of the cause of the ODS.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download