This article has been
cited by other articles in ScienceCentral.
Abstract
Giant cell fibroblastoma (GCF) is a rare soft-tissue sarcoma of fibroblastic origin. To the best of our knowledge, only one brief description of the MRI findings of GCF exists in the pathologic literature. Herein, we report a case of histologically proven GCF in a 3-year-old boy who underwent ultrasonography and MRI of a superficial mass in the abdominal wall.
초록
거대세포 섬유아세포종은 섬유아세포 기원의 드문 연조직 육종이다. 이제껏 우리가 알아본 바에 따르면 과거 병리학 논문에서 이 종양의 자기공명영상 소견에 대해 간략하게 언급한 기록이 있으며, 이것이 유일한 영상의학적 보고였다. 우리는 이번 증례에서 3세 남아의 복벽 표면에 나타난 거대세포 섬유아세포종의 조직학적 소견과 초음파, 자기공명영상에서 나타난 영상의학적 소견을 보고하고자 한다.
Keywords: Soft Tissue Sarcoma, Giant Cell Fibroblastoma, Magnetic Resonance Imaging, Ultrasound, Pathology
INTRODUCTION
Giant cell fibroblastoma (GCF) is a rare low-grade soft tissue sarcoma in dermal or subcutaneous layer. It was previously classified as skin tumor but now it belongs to soft-tissue sarcoma in 2013 WHO classification (
12). GCF is a histological variant of dermatofibrosarcoma protuberans (DFSP), that dominantly occurs during childhood, and more than half of cases are seen before the age of 5 years (
34). It characteristically presents as a small to medium sized, slowly growing, non-tender, dermal or subcutaneous mass, fixed to overlying skin in trunk wall, groin and axillary area (
56). Pathologic finding is characterized by the presence of multinucleated giant cells and pseudovascular spaces (
6).
Up to now, there is only one brief radiologic description of GCF by MRI on pathologic literature (
7), and here we describe a case of GCF in 3-year-old boy who underwent ultrasonography and MRI.
CASE REPORT
A 3-year-old boy presented with a superficial mass in left lateral aspect of lower abdominal wall that had persisted for two months. There was no history of trauma or pain. Initially, the mass was small and progressively increased up to 2.5 cm. Physical examination revealed a well-circumscribed soft tissue mass at left lower abdomen, measuring 2.5 × 1 cm in size. It was non-tender and the overlying skin was unremarkable.
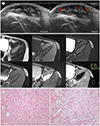
Ultrasound was performed to characterize the mass. Ultrasound demonstrated a 2.5 × 0.7 × 1.7 cm well-demarcated and oval shaped hypoechoic mass with irregular septum-like echogenic structures at the center in subcutaneous fat layer of left lower abdomen. There was prominent vascularity on Doppler study (
Fig. 1A). Based on the ultrasonographic findings, hemangioma was our first differential diagnosis. Then he underwent contrast-enhanced MRI for accurate localization of the mass. The subcutaneous mass involved the overlying skin but spared the underlying superficial fascia. The mass showed slightly high signal intensity on T2-weighted images (T2WI) compared to that of skeletal muscle and homogeneous intermediate to low signal intensity on fat-saturated T1-weighted images (T1WI). However, on fat-saturated T2WI, the areas of central low signal intensity and peripheral high signal intensity were separated. And there was intense peripheral enhancement of the mass on T1-weighted fat-saturated contrast-enhanced images. No diffusion restriction was identified (
Fig. 1B). There was no abnormal lymph node in scanned area, including both inguinal areas. We assumed the mass as benign soft tissue mass with a fibrotic component or a vascular tumor.
He underwent surgical excision to remove the mass and the histopathologic examination revealed diagnosis of GCF.
Macroscopically, the specimen was irregular, soft and white mass, which measured 2.2 × 2.0 × 0.8 cm, and on sectioning, there were multiple irregular dilated vascular channels. Microscopically, the mass demonstrated spindle cells and multinucleated giant cells scattered throughout the entire mass and slit-like pseudovascular spaces lined by the giant cells. Immunohistochemical staining showed diffuse positivity for CD34.
Interestingly, the histological composition differed between the center and the periphery of the mass. On low power image, the central portion showed hypocellularity of giant cells, spindle cells and sclerosis, which means abundant collagen. In contrast, the peripheral area of the mass showed hypercellularity of giant cells and spindle cells with abundant vascular channels and myxoid component (
Fig. 1C).
After 6 months from surgery, the patient had no recurrence of the mass in the left lower abdomen.
DISCUSSION
GCF was first described in 1982 when Drs Enzinger and Schmooker reported 20 cases of GCF in their seminal abstract (
5). GCF is a locally aggressive histological variant of DFSP that primarily affects children. GCF affects dominantly males during the first decade of life and more than half of cases are seen before the age of 5 years (
34). GCF and DFSP share common clinical features of male predominance, slow growth, painlessness, and superficial location of mainly trunk, and both are known to have identical gene fusion, translocation t (17;22). There also have been reports of hybrid tumors of them (
237).
In gross specimen, GCF appears as an unencapsulated, ill-defined, and infiltrative gray to yellow mucoid mass. It ranges from 1 to 8 cm in dimension with a mean of 3–4 cm (
35). Histopathologically, it characteristically shows pseudovascular spaces lined by spindle cells and multinucleated giant cells in a fibromyxoid to hyalinized stroma, not observed in DFSP. Most cases demonstrate CD34 positivity, which is suggestive of a specialized fibroblast on immunohistochemistry (
356).
There is one case report describing MRI findings of GCF in pathologic literature. In this report, GCF showed T2 high, and T1 low signal intensity, and marked contrast enhancement which is similar to our imaging findings. In our case, GCF showed slightly T2 high signal intensity compared to that of skeletal muscle, T1 intermediate to low signal intensity, and intense peripheral enhancement on MRI. Furthermore, we were able to deduce characteristic findings of GCF by correlating the radiologic and pathologic findings of our case. On MRI, the signal intensity and enhancement pattern interconnected with the different degrees of cellularity and histological component between center and periphery of the mass in our case. Typically, hypocellular lesion with abundant dense collagen results in decreased signal intensity on T2WI (
89). In our case, the relatively low signal intensity on fat-saturated T2WI was well correlated with mostly abundant collagen with hypocellularity in the central portion of the mass. On ultrasound, irregular septum-like echogenic structures was noted in corresponding area. In contrast, peripheral relatively high signal intensity on fat-saturated T2WI and hypoechogenecity on ultrasound were equal to the areas showing peripheral and intense enhancement on T1-weighted fat-saturated contrast-enhanced images. It seemed that this area is related to hypercellularity with abundant vascular channels and myxoid component of peripheral portion of the mass.
However, there is no pathologic report mentioned about degrees of cellularity and histological component between the center and the periphery of GCF. Jha et al. (
3) have reported that GCF ranged from solid and collagenized to angiectoid and myxoid component, and most of the GCF cases showed relatively hypocellular mass. Therefore, it is possible that the different degrees of cellularity and histological components between center and periphery of the mass can be seen, and so can the imaging findings. The radiologic findings in our case cannot be regarded as typical imaging features of GCF, and further studies are needed to establish the radiologic findings of GCF.
A differential diagnosis of GCF includes many soft tissue masses located in dermis and subcutaneous tissue. Firstly, DFSP would be the top differential diagnosis, because is a histological variant of GCF and the most common mesenchymal superficial malignancy. However, DFSP manifested as nonspecific MRI findings with low signal on T1WI and high signal on T2WI with variable enhancement, so that the differentiation of DFSP from GCF is difficult without pathologic confirmation. Secondly, hemangiomas are commonly encountered superficial hypervascular masses in clinical practice in all age groups. On MRI, hemangiomas may be distinguished from GCF that mass showed more high signal intensity on T2WI with lobulated contour and revealed phleboliths. Lastly, epidermal inclusion cysts can be a differential diagnosis by their location and tumor margin, however, easily distinguished from GCF in that they show no vascularity on Doppler ultrasonography nor enhancement on MRI.
Treatment options include removal of the tumor with wide excision. Despite the fact that local recurrence can be found in up to 50 percent of cases, no pure GCF metastasis has been reported (
36).
This is the first case report of a rare GCF with radiologic and pathologic correlation. Although GCF is difficult to diagnosis due to its nonspecific imaging findings and rarity, GCF can be proposed as one of the differential diagnoses when it appears to be superficially located soft tissue mass with well-defined margin in the trunk of a patient under the age of 5 years.