This article has been
cited by other articles in ScienceCentral.
Abstract
Inflammatory myofibroblastic tumor is a rare benign lesion that accounts for 0.04–1% of all lung tumors and usually appears as a solitary pulmonary nodule or mass. Here, we report the case of an endobronchial inflammatory myofibroblastic tumor in a 21-year-old man with a focus on the imaging findings and a review of previous literature.
초록
염증성 근섬유모세포 종양은 전체 폐 종양의 0.04~1%를 차지하는 드문 양성 종양이며 대개 고립성 폐결절이나 종괴로 보인다. 우리는 21세 남자 환자에서의 기관지 내 염증성 근섬유모세포 종양 증례를 영상소견을 중심으로 보고하고 관련된 문헌을 고찰하고자 한다.
Keywords: Myofibroblasts, Bronchial Neoplasms, Multidetector Computed Tomography
INTRODUCTION
Inflammatory myofibroblastic tumor (IMT) is a lesional pattern of inflammatory pseudotumor, which is histologically characterized by a mix of inflammatory cells such as plasma cells, lymphocytes, and eosinophils, and spindle cells (
1). It can occur in almost any part of the body, but is most commonly found in the lung, orbit, peritoneum, and mesentery. It tends to occur in people under 40 years of age with both sexes affected equally. It accounts for 0.04–1% of all lung tumors and usually appears as a solitary pulmonary nodule or mass (
2). Endobronchial location of IMT is rare and accounts for less than 12% of the reported IMT cases (
3). We report the case of endobronchial IMT in 21-year-old man, with a focus on imaging findings and review the previous literatures.
CASE REPORT
A 21-year-old man presented with abnormal findings of chest radiograph taken during a military medical examinations. He was asymptomatic and didn't have any abnormal findings on physical and laboratory examinations. He had a history of pneumonia at middle school.
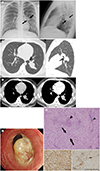
Chest postero-anterior and lateral radiographs showed a well-defined mass in left lower lobe (
Fig. 1A). Lung window images of computed tomography (CT) images demonstrated a 5 cm sized, well-defined, lobulated or branching mass with a small endobronchial portion (iceberg lesion) in the superior segment of left lower lobe (
Fig. 1B). Contrast-enhanced CT scan showed mild enhancement (20 Hounsfield unit) of the lesion (
Fig. 1C). There was no evidence of air-trapping, distal atelectasis or obstructive pneumonia. No significant peribronchial, hilar or mediastinal lymph node enlargement was seen. Bronchoscopy revealed a whitish, endobronchial tumor arising from the superior segmental bronchus of left lower lobe (
Fig. 1D). Bronchoscopic biopsy was performed to confirm the diagnosis of tumor and biopsy specimens revealed spindle cell neoplasm with myxoid background, but further confirmation was difficult. Therefore, he was planned for complete resection of the tumor and underwent video-assisted thoracoscopic left lower lobectomy. The resected tumor was grossly yellowish-white and fibrotic, measuring 5.1 × 2.7 × 4.6 cm in size and a well circumscribed peribronchial mass with endobronchial protrusion. Microscopic examination revealed proliferation of spindle cells showing fibroblastic and myofibroblastic differentiation and multifocal infiltrations of lymphoplasma cells in myxoid stroma (
Fig. 1E). Immunohistochemical analysis showed positive staining for vimentin and smooth muscle actin (
Fig. 1E). The tumor cells were not reactive to panCK, S100, ALK1, CD34, EMA, C-kit, GFAP, desmin, MUC-4 or CD21. Therefore, the tumor was confirmed as IMT.
The patient remained asymptomatic over 1 year after surgery and follow-up imaging studies showed no evidence of any residual or recurrent mass.
DISCUSSION
Diagnosis of IMT is difficult to establish before pathologic confirmation because of its diverse and non-specific imaging findings. It is typically seen as a well-defined, single nodule or mass with peripheral and lower lobe predominancy (
34). It can be multiple in less than 5% of cases (
4). On CT, IMTs have a variable and nonspecific appearance with a well-circumscribed, solitary, peripheral pulmonary nodule or mass. They usually appear with heterogeneous attenuation and enhancement. Calcification within the lesion can occurs more frequently in children (
345). On magnetic resonance (MR) images, IMTs were reported to have heterogeneous signal characteristics with a little higher signal than the muscle on T1- and T2-weighted images and show heterogeneous contrast enhancement as seen on CT (
6).
Airway involvement of IMT has been reported in 10–12% of all cases, up to 50% of cases (
237). Endobronchial IMT usually appears as an endobronchial polypoid nodule with or without extension beyond the bronchial wall. The possible hypothesis that IMT is closely related to the airway is that the tumor has originated from myofibroblastic differentiation of the primitive mesenchymal cells in the submucosal layer of the tracheobronchial wall (
8). As a result, the tumor may appear as an endobronchial nodule with an iceberg sign as shown in our case.
A systemic approach is needed for the differential diagnosis of large airway tumor or tumor-like conditions (
9). The most important step is to make sure that which airways are involved because the main site of tumor invasion depends on its type. In our patient, the tumor was located in lobar and segmental bronchi. Tumors predominantly affect lobar or segmental bronchi include squamous cell carcinoma, carcinoid, hamartoma, mucoepidermoid carcinoma, and metastasis. Squamous cell carcinoma is strongly associated with cigarette smoking and frequently has an intraluminal growth pattern that can cause airway obstruction and atelectasis. Endobronchial carcinoid tumor usually appears as a well-defined spherical or oval mass and strong contrast enhancement. On CT, endobronchial hamartoma may contain cartilage, fat, and fibrous tissue. Mucoepidermoid carcinoma usually occurs in younger patients and it usually appears as a small nodule or mass that may follow the branching pattern of the lobar bronchi. Airway metastasis is rare and appears as multiple discrete nodules or masses on CT. In our patient with young age, the tumor had low density with mild enhancement on contrast-enhanced CT. Therefore, we first considered mucoepidermoid carcinoma or hamartoma as a differential diagnosis. Imaging findings of IMT vary according to the tissue components, but usually they have heterogeneous enhancement on contrast-enhanced CT. If the tumor has a large amount of fibrous component, it may have little enhancement on late arterial phase (
10), but a strong contrast enhancement on the delayed phase. In our institution, enhanced images are usually obtained at 70 seconds after contrast medium injection. Therefore, if delayed CT or MRI were performed, it would have been helpful in differential diagnosis. PET-CT was not performed in our case, but if performed, it would be helpful to rule out malignant tumor.
In conclusion, although the prevalence of endobronchial IMT is low, IMT should be included in the differential diagnosis when a well-defined, polypoid, endobrochial nodule or peribronchial nodule with iceberg sign is seen on CT, especially in young adult.