This article has been
cited by other articles in ScienceCentral.
Abstract
Adenomyoepithelioma (AME) is a rare breast neoplasm composed of both epithelial and myoepithelial cells with biphasic proliferation. Although most AMEs are benign, malignant transformation of either or both cellular components may occur. This report describes an unusual rapid local tumor recurrence a month after excision of the myoepithelial carcinoma arising in an AME. Ultrasound and MRI showed small recurrent masses in the superficial part of a hematoma. This report suggests the benefit of immediate postoperative breast imaging in patients with malignant AME with potential for local recurrence, such as those with narrow resection margins or high mitotic activity.
초록
선근상피종은 관상피세포와 근상피세포의 이상성 증식을 특징으로 하는 유방의 매우 드문 질환이다. 대부분의 선근상피종은 양성이나 관상피세포 또는 근상피세포에서, 혹은 둘 모두에서 악성 변형을 보일 수 있다. 본 증례 보고는 선근피상종 내부에 발생한 근상피암으로 절제술 시행 1달 후에 빠르게 발생한 국소 재발에 관한 것이다. 초음파 검사와 자기공명검사에서 수술 후 생긴 혈종의 표재성 부분에 여러 개의 작은 재발 병소들이 관찰되었다. 본 증례 보고는 악성 선근상피종에서 수술 절제 면이 정상 조직과 가깝거나 높은 유사분열활성을 보이는 경우는 국소 재발의 가능성을 고려하여 수술 직후 유방영상검사 시행을 제안하고자 한다.
Keywords: Adenomyoepithelioma, Breast Neoplasms, Neoplasm Recurrence, Local, Ultrasonography, Magnetic Resonance Imaging
INTRODUCTION
Adenomyoepithelioma (AME) is a rare breast tumor formed of bicellular proliferation of epithelial and myoepithelial components. They occur at all ages but are more frequent in post-menopausal women. Although most AMEs are benign, malignant transformation can occur rarely in the epithelial component, myoepithelial component or both (
12). Malignant AME in the breast has the potential for tumor recurrence, and distant metastases and various organ metastases, such as the liver or lungs, have been reported (
34).
As this tumor is extremely rare, few studies or image findings have described the characteristics of AME. AME presents as a solitary mass without calcifications in breast imaging (
56). We report a case of rapid local tumor recurrence within one month after resection of malignant AME on the right breast.
CASE REPORT
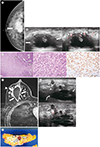
A 50-year-old woman presented with a palpable mass on the right breast. Mammography showed a 4 cm-sized, irregularly shaped, indistinctly marginated, hyperdense mass (
Fig. 1A). There was no associated calcification. B-mode ultrasound revealed an indistinctly marginated, irregularly shaped, complex cystic and solid echoic mass. Doppler ultrasound was performed with microvascular imaging and demonstrated centripetal, linear, and branching vascular flows (
Fig. 1B). We assessed the mass as suspicious for malignancy (category 4B) according to the Breast Imaging Reporting and Data System. Ultrasound-guided core needle biopsy was performed four times with a 14 G needle. Pathologic examination revealed an epithelial-myoepithelial neoplasm, and excisional biopsy was recommended because the possibility of malignancy could not be excluded. The excisional biopsy specimen was sectioned revealing a poorly demarcated whitish solid mass with cystic change, measuring 3.5 × 2.5 cm. The margins of the specimen were disease free, but the tumor was 1 mm apart from nearest surgical resection margin. Microscopic examination of the resected specimen showed that most of the tumor consisted of myoepithelial carcinoma cells with a minority of AME cells in the benign biphasic pattern. Some parts of the tumor showed aggregates of plasmacytoid myoepithelial cells with moderate nuclear atypia and high mitotic activity, which counted up to 28 per 10 high-power fields. The infiltrating tumor cells were positive for p63, supporting the myoepithelial origin (
Fig. 1C). The tumor cells were triple negative by immunohistochemistry (negative estrogen receptor, negative progesterone receptor, and negative human epidermal growth factor receptor 2), and the Ki-67 proliferation index was positive in 30% of cells.
One month after the excisional biopsy, the patient underwent breast dynamic MRI to plan further treatment. On MRI, some small enhancing masses appeared in the superficial part of a postoperative hematoma and showed homogeneous or rim enhancement (
Fig. 1D). The size of the larger mass was 1.4 × 0.6 cm and the size of the smaller mass was 0.7 × 0.5 cm. The masses showed early rapid and delayed washout enhancement. There was no abnormal lymphadenopathy. On ultrasound, new circumscribed or indistinct round hypoechoic masses appeared in the superficial part of the hematoma. They had centripetal, peripheral, linear or branching vascular flows on Doppler ultrasound (
Fig. 1E). We performed a core needle biopsy and confirmed these new masses as a local tumor recurrence. Finally, the patient underwent modified radical mastectomy after MRI and ultrasound examination two weeks later. On the pathologic examination, two recurrent malignant AME sized 1.8 × 1.2 cm and 1.3 × 0.9 cm were revealed and they were located in the superficial part of the hematoma (
Fig. 1F).
DISCUSSION
AME is a slow growing neoplasm in which there is biphasic simultaneous proliferation of ductal and myoepithelial cells. Most AMEs are cured by complete excision. However, one report described local invasion and recurrence as early as 4 months and as late as 23 years after resection of the primary tumor in both benign and malignant AMEs (
6). The present case had a rapid local tumor recurrence one month after resection.
Various causes of local recurrences after excision of AMEs have been reported. Hayes (
2) reported that local recurrence may be associated with the multinodular growth and intraductal extension of the primary tumor. Rosen (
7) suggested that local recurrence frequently occur due to incomplete excision or persistent intraductal foci. High mitotic activity was suggested as one of the causes of local recurrence in myoepithelial lesions of the breast including AME by Tavassoli (
1). In our case, the pathologic examination revealed that the margins of the initial excisional biopsy specimen were disease free, but the tumor margin was very close to the resection side (1 mm). In addition, our case had a high mitotic count and high Ki-67 level. Therefore, the rapid local recurrence in our case may be associated with a narrow excision margin and high mitotic activity in the primary tumor.
Clinically, AME presents as a palpable mass and rapid enlargement of a mass is highly suggestive of malignant change (
8). The imaging feature of AME is a nonspecific solid mass (
68). On mammography, AME usually appears as a round, oval, or lobulated hyperdense mass with circumscribed or indistinct margins and no calcification (
6). On ultrasound, AMEs usually present as solid hypoechoic masses with non-circumscribed margins (
9). Some AMEs appear as complex cystic and solid echoic masses, which can occur due to the proliferation of neoplasms that are compressed or obstructed in adjacent duct spaces. Malignant AMEs tend to have indistinct margins, posterior shadowing, and marked architectural distortion (
8). An MRI can provide evidence of additional hemodynamic characteristics. Malignant AME tends to have a washout enhancement pattern (
6). In our case, the mass had suspicious imaging features, including non-circumscribed margins and complex cystic and solid echo pattern on the initial ultrasound examination. Therefore, we considered the possibility of a papillary or mucinous tumor. The recurrent masses appeared as round hypoechoic masses on ultrasound and demonstrated washout enhancement on MRI. The recurrent tumors were located in the superficial part of a hematoma, and these may have occurred along the initial core needle biopsy tract. Chao et al. (
10) reported a local recurrence of breast cancer in a core needle biopsy site. The core needle biopsy site can be a potential area of malignant seeding and local recurrence, and Chao et al. (
10) recommended resection of the core biopsy tract at the time of definitive surgery of the primary tumor.
In conclusion, AME is a rare breast neoplasm composed of both epithelial and myoepithelial cells with biphasic proliferation. Patients with AME should be aware of the potential for recurrence after local excision in both benign and malignant tumors. Immediate postoperative breast imaging is recommended for patients with malignant AME whose tumors have incomplete or narrow resection margins, or high mitotic activity with the potential for local recurrence.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download