CASE REPORT
A 56-year-old male patient visited gastroenterology outpatient department for ongoing weight loss, anorexia, and abdominal discomfort for 3 months. He had no past medical history and no recent medication history. He complained mild abdominal discomfort on right upper quadrant worsening when he lay down on back. He had lost his body weight from 75 kg to 55 kg during last 3 months. Vital sign and general physical examination was unremarkable. Initial laboratory tests revealed normocytic normochromic anemia as decreased hemoglobin count of 9.3 g/dL (normal range: 13–17 g/dL) and also made suggestion of biliary disease as increased alkaline phosphatase (ALP) of 133 U/L (normal range: 40–129 U/L). On the initial urine analysis, pyuria of 10–19/HPF white blood cell (normal range: 0–4/HPF) and bacteriuria of 30/HPF (normal value: 0) were detected.
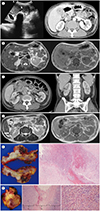
Abdominal ultrasonogram revealed a 2.9 cm nodular stone in the lumen of GB with diffuse mass-like mural thickening. Intramural low- or anechoic portions were also identified (
Fig. 1A). On abdominal CT, GB showed diffuse wall thickening with a few intramural nodular low-attenuations (
Fig. 1A). Pericholecystic hyperemic change was noted along GB bed of hepatic parenchyma. XGC was considered and the possibility of tumorous condition such as adenocarcinoma was also considered. MRI of upper abdomen demonstrated an oval GB stone and diffuse wall thickening as heterogeneous intermediate signal intensity (SI) on T1-weighted images (T1WI) with intramural irregular cystic change (
Fig. 1B). At right renal cortex, a focal soft tissue density with fuzzy margin was found in CT with intralesional non-enhancing low attenuated portion (
Fig. 1C). Renal cortical abscess, hemorrhagic or infected cyst as well as tumorous lesion was considered. This lesion on MRI showed a cystic lesion with low SI rim on T2WI (
Fig. 1D). For pathologic diagnosis as well as removal of GB and renal lesions, the patient was referred to hepatobiliary surgery department and underwent cholecystectomy and partial right nephrectomy. During operation, contracted GB with severe adhesion to transverse colon, duodenum, and omentum was found. GB wall was diffusely irregularly thickened. After confirming that it was not GB cancer, XGC and XGP by intraoperative frozen section biopsy, entire GB with local adhesive lesion to the transverse colon and the lateral cortex of the mid to upper portion of the right kidney with adhesive perirenal tissue were resected.
In pathologic examination, all specimens revealed chronic active XGI, with absence of any pyogenic abscess, tuberculosis, carcinoma or other neoplasm. The GB is disfigured with prominent irregular mural fibrous thickening, multifocal extensive mucosal ulcers, transmural old hemorrhages and multiple yellowish xanthogranulomatous nodules or plaques (
Fig. 1E), with a large cholesterol stone. Microscopically, yellowish nodules are composed of nodular aggregates of foamy histiocytes, containing abundant lipid droplets or hemosiderin granules, admixed with severe polymorphic lympho-reticular inflammatory cells and fibroblasts were noted in GB wall (
Fig. 1E), transverse colonic subserosal & serosal fibroadiopose tissue and right renal cortex (
Fig. 1F) and perirenal fibroadipose tissue, consistent with XGI. His final diagnosis was 1) chronic active XGC with focal mural perforation and local invasion to omentum and subserosa of transverse colon and 2) sequentially developed the focal type of chronic active XGP. He was treated with intravenous ciprofloxacin and discharged as symptoms subsided.
DISCUSSION
XGI, characterized by aggregation of lipid-laden foamy macrophages, usually occurs at GB and kidney but some rare histiocytoses or genetic diseases can be considered when other atypical portions of body are involved in young patients (
1). Most XGCs are preceded by calculous or tumorous biliary obstruction. In the same way, most of XGP is caused by chronic urinary tract obstruction presenting as diffuse type involving entire kidney whereas only 10–15% presents as focal type without relevance to urinary obstruction. Pathophysiology of focal type XGP is still not clear (
2). In the presented case, focal type XGP was incidentally founded along with typical XGC, and such case has never been reported in English literature.
In our case, focal type XGP can be explained by two mechanisms; secondary involvement from XGC or independent manifestation as systemic process. As XGI tends to involve neighboring organs, XGC has been reported to infiltrate to extrahepatic bile duct, stomach, duodenum, colon, and liver and to make fistula toward skin (
1). For us, adjacent invasion of XGC was histologically confirmed in omentum and transverse colon. Also, along the inferior aspect of right hepatic lobe, infiltrative density was featured in CT, having adjacency with right side focal type XGP (
Fig. 1C). Retroperitoneal extension of GB pathology could be explained when posterior pericholecystic erosion and posterior parietal peritoneum is infiltrated (
3). Even in case of XGC with xanthogranulomatous pseudotumor at left side diaphragm, localized infiltration through potential anatomic route rather than remote seeding was considered (
4).
In the latter hypothesis, on the other hand, focal type XGP can develop independently from XGC. Multifocal systemic XGI can be found in histiocytic process such as Erdheim-Chest disease, Rosai-Dorfman disease, hemophagocytic lymphohistiocytosis, and juvenile xanthogranuloma. Inherited lysosomal disorders also result systemic XGI. However, these diseases usually affect young age patients at different target organs like bones and vessels (
1). In the presented case, such organs were clinically and radiologically intact. Moreover, related systemic pathology such as bleeding tendency and dyslipidemia were not observed in the laboratory analysis. In histopathologic examination, focal type XGP seemed to protrude inwardly from perirenal tissue toward renal cortex (
Fig. 1F). Therefore, we assumed our focal type XGP as secondary invasion from primary XGC more likely than multifocal systemic involvement. This case is meaningful to propose a new pathogenesis of focal type XGP. Secondary focal type XGP was previously described only in one case so far, originating from duodenal diverticulum (
5).
Our case is also valuable in that it shows unprecedented MRI findings of focal type XGP. Known MRI findings of focal type XGP include usual cystic nature of cavitary fluid with possible fluid-fluid level. Cavitary border can be seen as intermediate SI on T1 and T2WI and may enhance when introduced with intravenous gadolinium. If present, perirenal strands can be hypointense on T1 and T2WI (
6). Our case showed a cavitary lesion of slightly high SI with rim-like hypointensity on T2WI. Considering chronicity of our case, rim-like T2-shortening may be related to hemorrhage and fibrosis (
7). Pathologic analysis found multistage hemorrhage with hemosiderin-laden histiocytic accumulation and pericapsular organizing fibrosis (
Fig. 1F). Because peripheral low SI in T2WI is a nonspecific finding, renal abscess and renal tumor with central necrosis should be on the list of radiologic differential diagnosis. On T1WI, central portion of the lesion was indistinguishable with renal parenchyma, suggesting protein-rich fluid. In contrast to T2WI, peripheral portion on T1WI was isointense, assuming that presence of xanthoma cell may compensate for T1-lengthening effect of hemosiderin and fibrosis (
8).
Clinical information of the patient could make better diagnosis but had limited value in this case. When compared with GB cancer, the most important differential diagnosis of XGC, abdominal pain, fever, and jaundice are more frequent in XGC patients. Leukocytosis, serum bilirubin, serum ALP and weight loss were invalid to differentiate between XGC and GB cancer (
9). Weight loss is more frequently observed in XGP than in XGC and other usual symptoms of XGP include fever and flank pain (
2). Considering size and severity, it was reasonable to regard weight loss and abdominal pain of our patient as result of XGC. Although pyuria and bacteria are also common in XGP, renal lesion of the presented case seemed too subtle for these urine abnormalities which were possibly due to superimposed acute pyelonephritis. In absence of fever and leukocytosis as in our case, radiologic finding played crucial role in making diagnosis.
In conclusion, focal type XGP can develop in association with XGC as localized infiltration. In focal type XGP, MRI may depict focal infiltrative cystic lesion with peripheral low SI rim in T2WI, resulting from hemosiderin and fibrosis.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download