INTRODUCTION
Muscular torticollis is clinically characterized as a head tilt, limited neck rotation, and a palpable mass in the involved sternocleidomastoid (SCM) muscle (
1). Ultrasonography (US) has been used to screen neck masses and to support clinical diagnoses of congenital muscular torticollis (
2). On the other hand, real-time sonoelastography (RTS) is a recently developed US-based technique that can assess the mechanical properties of soft tissues by measuring their stiffnesses or elasticities (
3). And thus, it might be useful for assessing muscle elasticity to complement conventional B-mode US.
Usually, to evaluate treatment effectiveness during physiotherapy of muscular torticollis, physiatrists assess the neck rotation and side flexion deficits by the physical examination. However, these examinations are not always perfect due to variable expertness of doctors or patient's condition by very young age. And thus more objective methods are sometimes necessary and US is the most commonly recommended method. When we undergo follow-up US examinations in patients with congenital muscular torticollis, most radiologists measure SCM muscle thickness and evaluate its echogenicity using conventional B-mode US. However, sometimes, the results of grayscale images such as change in the thickness of the thickened muscle do not match clinical and physical examination findings (
4). We have occasionally encountered cases in which it is difficult to determine progress based on the thickness or echogenicity of SCM muscle. And thus, conventional US has limitations for the evaluation of congenital muscular torticollis.
To our knowledge, no previous study has examined the clinical efficacy of follow-up RTS comparing with physical examination for evaluating the effectiveness of physiotherapy, although some previous studies (
56) have shown the diagnostic feasibility of sonoelastography in patients with congenital muscular torticollis. This study was undertaken to evaluate the clinical efficacy of RTS for the follow-up of congenital muscular torticollis based on measurements of SCM muscle elasticity and to determine whether these measurements are correlated with clinical or physical results.
MATERIALS AND METHODS
This study was approved by our Institutional Review Board. Because this study was retrospective analysis, informed consents were not needed (IRB No. E-2015069).
PATIENTS
The B-mode US and strain RTS findings of 152 infants (79 males, 73 females; age at first examination, ≤ 12 months) suspected to have torticollis, including head tilt, limited neck rotation or a palpable neck mass obtained from November 2012 to December 2014 were prospectively examined. Infants who did not show asymmetrical thickening or abnormal echogenicity of SCM muscle at initial US examination were excluded. And also, infants with a congenital anomaly of the cervical spine, a clavicular fracture, spasmodic torticollis, or neurogenic or ocular torticollis were excluded, as were infants with suboptimal image quality due to crying or excessive movement or lacking sonoelastography images. Infants with congenital SCM torticollis, as determined by a physiatrist (an expert in pediatric rehabilitation medicine) and radiologist, and who underwent follow-up US examinations, which included RTS more than twice were enrolled. At initial examination, all infants performed grayscale US examination and sonoelastography, and had not undergone any form of medical treatment, such as, physiotherapy. Finally 34 infants (23 males, 11 females; age range at initial examination, 2 days–3 months; mean days, 33.8 days) were included in this study. There were no infants with bilateral involvement of muscular torticollis. Eighteen infants underwent follow-up US examinations twice, 13 three times, and the remaining 3 four times (a total of 87 examinations). Total follow-up period did not exceed 12 months in each patient.
US EXAMINATION
One radiologist with 11 years of experience at musculoskeletal US performed B-mode US and sonoelastography of strain type by using a commercially available ultrasound system equipped with a 12-5 MHz linear transducer (iU 22, Philips Medical System, Eindhoven, the Netherlands). Sonographic examinations were performed with patients comfortable and stable, unsedated, in a supine position with slight rotation of the head to the contralateral side and with neck extension with the aid of parents or nurses.
Longitudinal views of bilateral SCM muscles were scanned without probe compression several times in each infant. The examiner made efforts to scan with the probe moving parallel to SCM muscle.
After B-mode US examination, the same radiologist performed sonoelastography along the long SCM axis using the same infant and transducer position performed for B-mode sonography. The conventional grayscale image was displayed on the right side of the screen and the color coded RTS image on the left side. The compressive force applied to the SCM muscle using a free hand technique using a conventional transducer was adjusted, according to an indicator on the video screen, that showed appropriate strain at the region of interest (ROI). Compression was applied in a vertical direction to long-axis of SCM.
The rectangular ROI in the strain image covered the entire SCM muscle and sufficient surrounding normal tissue. Colors showed relative tissue stiffnesses within the ROI and ranged from red (high elasticity), green (medium elasticity) to blue (low elasticity). Elastographic images were obtained in dynamic, real time during tissue compression and decompression over at least three compression-relaxation cycles and a video of the process was recorded in the internal memory ultrasound device.
IMAGE ANALYSIS
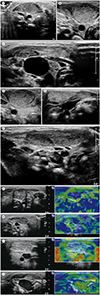
Two radiologists retrospectively evaluated the US images transferred to a picture archiving and communication system in consensus. Echogenicities, morphologies, and SCM muscle thicknesses were analyzed on B-mode sonograms. Involved SCM echogenicities were arbitrarily classified with homogeneous hyperechoic (score 0), heterogeneous hyperechoic ≥ 50% (score 1), heterogeneous hyperechoic < 50% (score 2), and iso-echoic (score 3) as compared with normal muscle (
Fig. 1A–C).
Morphologies of involved SCM muscles were interpreted as focally mass-like, fusiform, and overall thickened shapes (
Fig. 1D–F). Thicknesses of involved and contralateral SCM muscles were measured at the thickest point several times (3–5 times) and the median values were used in the analysis. In addition, differences between the thicknesses of the involved and contralateral SCM muscles were calculated.
Stored sonoelastogram cine clip files were replayed to select most representative images, based on the compression bar indicator of tissue deformation level. Elasticity can be represented by color coding (qualitative) or by strain ratio (quantitative). The color scale ranged from red (soft: high elasticity) to blue (rigid: low elasticity). Green indicated the elasticity between red and blue. The qualitative elastic pattern was arbitrarily graded using four point system according to the color scale as follows: score 0 (nearly homogeneous blue: inelastic), score 1 (mainly blue with small areas of green < 50% in the ROI, slightly elastic), score 2 (blue and green areas with nearly the same distribution of blue and green or green areas accounting ≥ 50% of the ROI, moderate elastic), and score 3 (nearly homogeneous green or green to red: highly elastic) (
Fig. 1G–J).
Quantitative analysis was performed using strain ratios, which were measured to assess the relative stiffnesses of involved SCM muscle and adjacent subcutaneous fat layer in a selected ROI. Strains were quantified by placing an ROI to cover the entire involved SCM region using a free-hand technique and placing a small round ROI in adjacent subcutaneous fat. After applying ROIs, strain ratios (involved SCM muscle versus adjacent subcutaneous fat) were calculated automatically using software (Q-Lab software, Philips Medical Systems).
CLINICAL EXAMINATIONS
One rehabilitation doctor classified neck rotation and side flexion deficits using 5 grades according to angles of deviation by chart review. The grading system was as follows: grade 0, no deficit; grade 1, deficit ≤ 5 degrees; grade 2, deficit of 5–15 degrees; grade 3, deficit of 16–30 degrees; grade 4, deficit ≥ 30 degrees. Neck rotation limitation and side flexion deficit were examined in the supine position at the time of impossible neck control and sitting position at the time of possible neck control.
During follow-up, a physiotherapist specially trained in pediatric neuromuscular disorders performed a manual stretching treatment for all infants 3 times per week. In addition, parents were trained to perform a home program involving proper positioning and passive stretching.
STATISTICAL ANALYSIS
Linear regression analysis and Spearman correlation coefficients were used to evaluate relationships between neck rotation and neck side flexion deficits and morphology, echogenicity, qualitative (color score) and quantitative elasticity (strain ratio) of involved SCM muscles and differences between involved and contralateral SCM muscle thicknesses. Also, we used a two-sample t-test to determine the difference in the thickness of the SCM muscle between involved and contralateral side. The data analysis was performed using statistical software (IBM SPSS statistics for Windows, version 21; IBM Corp., Armonk, NY, USA). Statistical significance was accepted for p values < 0.05.
RESULTS
The mean interval between each follow-up US examination was 105 days (range, 3 months–4 months 15 days) and the last follow-up US examinations did not exceed one year after the first US. Twenty-two infants had right SCM muscle torticollis and the other 12 had left torticollis.
The grades of neck rotation and side flexion deficit are summarized in
Table 1. Of the three patients that underwent three follow-up US examinations (not seen in Table), two had grade 2 neck rotation deficits and one grade 0. And for side flexion deficits, two were of grade 1 and one was of grade 2. Most infants showed a reduction in the grade after physiotherapy.
The thicknesses of involved and contralateral SCM muscles at initial and follow-up examinations are presented in
Table 2. Minimum, maximum and median values were obtained for each patient. Variations in involved SCM muscle thicknesses were greater than those of contralateral SCM muscle thicknesses. At each examination, the median values of SCM thicknesses showed significant differences between involved and contralateral sides. The mean values of differences between median values of involved and contralateral SCM muscle thickness were 5.73 mm (1.4–10.9 mm) at initial examinations, 3.12 mm (0.1–8.8 mm) at 1st follow-up examinations, and 2.19 mm (0–7.4 mm) at 2nd follow-up examinations.
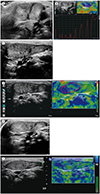
Regarding SCM muscle morphology evaluations, 25 patients had a focal mass-like appearance (
Fig. 2A), 8 a fusiform appearance, and the remaining one an overall thickened muscle at initial examinations. At 1st follow-up examinations, 8 had a focal mass-like appearance (
Fig. 2C), 15 a fusiform appearance, and 11 an overall thickened muscle. At 2nd follow-up examinations, 11 had a fusiform appearance (
Fig. 2E) and 5 an overall thickened muscle. Of the three patients that underwent 3rd follow-up examinations, two had a fusiform and the other one had an overall thickened muscle.
Echogenicities of involved SCM muscles at initial and follow-up examinations are shown in
Table 3. Of the three patients that underwent 3rd follow-up examinations, two showed homogeneous hyperechogenicity and the other one < 50% hyperechogenicity.
Elasticities according to the color scale (qualitative) are summarized in
Table 4. Of the three patients that underwent 3rd follow-up examinations, two had an elasticity score of 3 and the other had a score of 2. The mean values of strain ratio (quantitative) were 2.86 (2.25–3.37) at initial examination, 1.73 (1.05–2.38) at 1st, 1.54 (1.33–1.76) at 2nd, and 1.35 (1.27–1.51) at 3rd follow-up examinations.
Linear regression analysis (
Table 5) showed the difference between the thicknesses of involved and contralateral SCM muscles, and SCM muscle morphology, elasticity score (as determined by color patterns), and strain ratios of involved SCM were significantly correlated with grades of neck rotation deficit and side flexion deficit (
Fig. 1). Spearman correlation coefficient analysis (
Table 5) showed that all analyzed contents were significantly correlated with the results of clinical and physical examinations (
p < 0.0001). Elasticity score of the involved SCM showed the strongest correlation.
DISCUSSION
US is the imaging modality of choice for evaluating congenital muscular torticollis, and has been shown to visualize fibrotic change and mass formation in the SCM muscle (
5). Common US findings include a heterogeneous mass and fusiform thickening of the SCM muscle (
78). Initial therapy for an infant with congenital muscular torticollis consists of physiotherapy involving passive and active exercises and massage (
5). In this study, neck rotation deficit and side flexion deficit were found to be decreased in many patients who received physiotherapy. However, these physical examinations may not be performed properly according to the physiatrist's skill or insufficient cooperation of infants. Therefore, for assessment of treatment effect, more objective evidence may be needed in addition to physical examination. US is the most optimized imaging modality for evaluation of SCM muscle.
We usually measure the thicknesses of bilateral SCM muscles and use the difference between the thicknesses of involved and contralateral SCM muscles to evaluate the congenital muscular torticollis regardless of initial diagnosis or follow-up US examination. However, sometimes, decisions regarding improvements are difficult because thickness measurements are altered by respiration and swallowing, and by the degree of compression caused by the US probe during an examination. These thickness variations can cause confusions especially during follow-up examinations. Also in our study, variations in involved SCM muscle thicknesses were greater than those of contralateral SCM muscle thicknesses. Furthermore, the difference between involved and contralateral SCM thicknesses was found to be significant at each follow-up examination. Therefore, in determining the therapeutic effect, the simple thickness measurement of muscles seems to have limitations.
Regarding involved SCM muscle morphologies, 25 of the 34 patients (73.5%) showed a focal mass-like form at initial examination, and proportions with a fusiform or overall thickened muscle increased in follow-up examinations. However, morphology assessments were subjective and sometimes opinions varied during a single examination, although the morphology was found to be significantly correlated with physical examination findings in this study.
Histological studies of resected surgical specimens have demonstrated atrophy and fibrosis of muscle fibers in congenital muscular torticollis (
9). Cheng et al. (
10) suggested that the hyperechogenicity remains the most striking and reliable feature that is correlated with the severity of congenital muscular torticollis. However, hyperechogenicity was visible to variable extents at follow-up US examinations during physiotherapy. Echogenicity scores were not significantly correlated with the progress of SCM torticollis, although the extent of hyperechogenicity decreased in accord with improvements in neck rotation deficits or side flexion deficits.
Although conventional US provides information about muscle thickness, morphology, and echogenicity, these informations might not be enough to evaluate therapeutic response, and thus we sought to assess muscle stiffness using RTS. Sonoelastography allows qualitative and quantitative measurements of tissue elasticity, and has been reported to detect differences in muscle elasticity (
111213). Elastography of strain type is based on the principle that an externally compressive force is applied to the tissue causing axial tissue displacement (strain), and is calculated by comparing echo signal sets obtained before and after the application of mechanical stimulation, measuring the strain in one area relative to another and displaying results as a map (
14). Therefore, it is assumed that tissue displacement by compression is lower for rigid tissues than for elastic, soft tissues (
15). Tissue hardnesses differ for fat, muscles, and tendons, and also seem to reflect disease-induced change (
16). And thus, we evaluated changes in elasticity of SCM muscles during physiotherapy in infants with congenital muscular torticollis. We found that elastographic scores and strain ratios were strongly associated with physical examination findings.
Color scale sonoelastography provides an objective means of estimating color-coded graphic representations of the relative stiffnesses of structures within a selected ROI (
5). Standard color scale ranges are equipment manufacturer dependent. For example, red may represent elastic or rigid components on different machines. On our machine, red represented the most elastic components, and blue the most rigid component. Also, we measured strain ratio in this study using a software (Q-lab, Philips Medical Systems) for quantitative evaluation. However, strain ratio measurements are semi-quantitative, because they are the ratios of relative strains in areas of interest and reference areas (usually fat) (
17). Furthermore, strain ratio values are highly sensitive to ROI size and location. And thus, the values might be unreliable and poorly reproducible. Therefore, a more objective quantification method will be needed.
Several previous studies (
256) have focused on evaluating elastography as an initial diagnostic tool of congenital muscular torticollis. However, we assessed changes in SCM muscle elasticities and correlations between these changes and the results of physical examinations during physiotherapy. Both qualitative results based on color scale assessments and semi-quantitative results based on strain ratios were found to be significantly correlated with physical examination findings.
This study has several limitations that warrant consideration. First, the number of included patients was relatively small. Although 157 patients were examined during the study period, only 34 patients (87 examinations) were enrolled, because we included only patients that underwent RTS regularly during follow-up and excluded non-muscular torticollis. Regular RTS examination in infants was difficult due to crying or excessive movement. Second, because RTS results were obtained by manual compression using the freehand technique, reproducibility is an issue. To reduce this, we used a visual indicator of reliability on the video screen to obtain results at optimal strain. In addition, we avoided applying excessive pressure to the probe and applied the probe vertically. Third, a comparison with shear-wave sonoelastography results was not possible, because only the strain type was available in this machine. Quantitative assessment using the strain scale can be used only as a comparative index rather than as an absolute measurement of strain (
1618), and thus, in future, a comparison between the strain and shear-wave sonoelastographic techniques is needed. Fourth, the size of the ROI window used for sonoelastography is also an issue. The hardness of target tissue is influenced by window size, for example, a larger window that includes more surrounding soft tissue makes hard tissue seem harder, and a smaller window makes hard tissue appear softer. To reduce variabilities by this effect, we standardized window depth and width for examinations, and used a rectangular ROI in strain images that covered the entire SCM muscle including sufficient surrounding normal tissue.
In summary, color scale grades and strain ratios obtained with RTS were found to be more significantly correlated with clinical and physical examination findings than conventional grayscale US results, including echogenicity, morphology, and differences between involved and contralateral SCM thicknesses in patients with congenital muscular torticollis. Therefore, sonoelastography might raise confidence when evaluating the physiotherapy response of congenital muscular torticollis, and complement conventional B-mode US images.






 PDF
PDF ePub
ePub Citation
Citation Print
Print






 XML Download
XML Download