Abstract
Purpose
To investigate the clinical manifestations and prognosis of eyes with cilioretinal artery sparing central retinal artery occlusion (CRAO).
Methods
A retrospective study was conducted on 90 eyes diagnosed with complete CRAO, including 16 cases of cilioretinal artery sparing CRAO. Clinical features, visual outcome, papillomacular bundle involvement, and remnant visual field were analyzed according to cilioretinal artery sparing.
Results
Among eyes with complete CRAO, the proportion of cilioretinal artery sparing CRAO was 17.8% (16 / 90). Mean initial best-corrected visual acuities (BCVAs) (2.04 ± 0.69 vs. 2.34 ± 0.47, p = 0.039) and final BCVAs (1.65 ± 0.87 vs. 2.22 ± 0.84, p = 0.001) were significantly better in eyes of the cilioretinal artery sparing group than the non-sparing group. The proportion with poor visual outcome (final BCVA <20 / 200) was 81.3% in the cilioretinal artery sparing group and 97.3% in the non-sparing group (p = 0.01). In sub-group analysis within cilioretinal artery sparing CRAO eyes, ischemic involvement of the papillomacular bundle at disease onset was significantly more frequent in the poor vision group (BCVA <20 / 200, 12 / 13 [92.3%]) than in the good vision group (BCVA ≥20 / 200, 1 / 3 [33.3%], p = 0.016) and it was associated with preserved central visual field.
Conclusions
Although cilioretinal artery sparing is common in CRAO and has a better prognosis than complete CRAO, the visual outcome is generally poor and only a small proportion of eyes has preserved small central visual field. Ischemic injury of the papillomacular bundle at the acute stage of CRAO correlates with poor visual outcome and could be a prognostic sign.
Central retinal artery occlusion (CRAO) is an ocular vascular occlusive disease with very poor prognosis. In previous studies, Varma et al. [1] reported that only 10% of patients with spontaneous reperfusion experienced meaningful vision recovery. CRAO is the ocular analogue of cerebral stroke. The same atherosclerotic risk factors that predispose to cardiovascular, peripheral vascular, and cerebrovascular disease are present in CRAO, and these must be actively evaluated to prevent further medical comorbidities [2]. Effective treatment of CRAO must target acute reperfusion of the CRAO and prevention of ocular complications and further end-organ ischemia [1].
CRAO can be classified into 4 distinct clinical entities: non-arteritic CRAO, non-arteritic CRAO with cilioretinal artery sparing, arteritic CRAO associated with giant cell arteritis, and transient non-arteritic CRAO [3]. Although the inner half of the sensory retina derives its blood supply from the central retinal artery, in many cases a cilioretinal artery may supply a small portion of the retina around the optic disc. Cilioretinal arteries have been histologically shown to originate from short posterior ciliary arteries and, in rare instances, directly from the choroidal vessels [4]. The temporal cilioretinal artery may spare the fovea in some cases of CRAO. Therefore, cilioretinal artery sparing is important for protecting the macula and preserving good visual prognosis in CRAO [5]. It is generally believed that visual outcome after CRAO is better in the presence of a patent cilioretinal artery, which bypasses the occlusion site in the central retinal artery [4]. However, there have only been several case reports of cilioretinal artery sparing CRAO [67]. The incidence, clinical features, and visual outcomes of cilioretinal artery sparing CRAO have not been well studied and knowledge about cilioretinal artery sparing CRAO is currently insufficient.
The purpose of this study was to investigate the clinical manifestations and visual prognosis of cilioretinal artery sparing CRAO.
This study was approved by the institutional review board of Seoul National University Bundang Hospital (B-1905/540-110). Informed consent was waived due to the retrospective nature of the study. Initially, 209 eyes of patients with CRAO patients who visited our Ophthalmology Outpatient Clinic between 1 January 2003 and 31 March 2019 were reviewed. Patients diagnosed as acute non-arteritic complete CRAO with a follow-up period of ≥1 month were included in this study as cilioretinal artery sparing was only found in eyes with complete CRAO. Cases with iatrogenic causes (e.g., filler injection, intraocular surgeries; n = 27), combined ocular pathologies (e.g., proliferative diabetic retinopathy, age-related macular degeneration; n = 29), trauma (n = 3), follow-up period <1 month (n = 21), and incomplete CRAO (n = 39) were excluded. In total, 90 eyes form 90 patients with complete CRAO were finally included for the analysis.
Data pertaining to patient age, sex, and best-corrected visual acuity (BCVA) was obtained. Snellen visual acuity measurements were converted into logarithmic minimum angle of resolution (logMAR) equivalent values for statistical analysis. The ophthalmic examination included slitlamp biomicroscopy, indirect ophthalmoscopy, fundus photography, fluorescein angiography, optical coherence tomography (OCT; Spectralis OCT, Heidelberg Engineering, Heidelberg, Germany), and Goldmann visual field perimetry. Initial OCT images were compared with final OCT images to assess quantitative and qualitative changes in the retinal structure. If patients showed abrupt improvement in visual acuity at the follow-up visit, Goldmann visual field and BCVA were carefully re-measured to rule out the possibility of extra-foveal fixation.
The papillomacular bundle is a collection of retinal nerve fibers that carries information from the macula (central retina) to the optic nerve. To analyze the inner retinal structure, OCT images of horizontal scans of the foveal center including the papillomacular bundle area (nasal macula) and the temporal macula were analyzed. With these OCT images, inner retinal structural changes were evaluated by assessing inner retinal thickness, inner retinal hyper-reflectivity, and loss of layer-by-layer integrity. Inner retinal thickening was defined as increased thickness compared with corresponding areas of the contralateral unaffected eye. Inner retinal hyper-reflectivity was defined as increased reflectivity compared with adjacent normal retinal areas. Loss of layer-by-layer integrity was defined as indistinguishable borders or loss of layers due to ischemic injury [8].
All statistical analyses were performed using IBM SPSS Statistics ver. 22 (IBM Corp., Armonk, NY, USA). Fisher's exact test was used to examine between group differences in non-continuous variables (given unequal sample size). Mann-Whitney U-test was used for continuous variables, which were expressed as mean ± standard deviation. A p-value of <0.05 was considered statistically significant in all tests.
Of 90 eyes with complete CRAO, 16 eyes showed cilioretinal artery sparing (17.8%). A representative case with cilioretinal artery sparing CRAO is shown in Fig. 1A–1D and 2A–2C. Table 1 presents demographic and clinical characteristics of study subjects (16 cases of cilioretinal artery sparing CRAO and 74 cases of non-sparing complete CRAO). Mean age (67.00 ± 11.34 vs. 63.01 ± 16.09) and sex ratio (male ratio, 56.2% vs. 58.1%) were not different between the 2 groups. Mean initial BCVA and mean final BCVA were significantly different between cilioretinal artery sparing CRAO and non-sparing complete CRAO groups (2.04 ± 0.69 vs. 2.34 ± 0.47, p = 0.039; 1.65 ± 0.87 vs. 2.22 ± 0.84, p = 0.001) (Fig. 3). The proportion with poor visual outcome (final BCVA <20 / 200) was 81.3% in the cilioretinal artery sparing group and 97.3% in the non-sparing group (p = 0.01). Mean initial macular thickness was significantly different between groups and was thicker in the non-sparing complete CRAO group (355.19 ± 115.11 vs. 454.86 ± 152.56, p = 0.018). Mean final central macular thickness was greater in the cilioretinal artery sparing group than in the non-sparing group, but without statistical significance (233.73 ± 39.64 vs. 212.56 ± 32.78 µm, p = 0.075). Systemic characteristics associated with CRAO, such as diabetes mellitus, hypertension, stroke, carotid stenosis, and cardiac disease, were not significantly different between the two groups.
Demographic and clinical features of the 16 cilioretinal artery sparing CRAO cases are shown in detail in Table 2. Among all 16 patients, 10 patients had hypertension, three patients had previous stroke history, and six patients had carotid stenosis. Two patients had coronary artery disease, two patients had hyperlipidemia, two patients had angina, and two patients had atrial fibrillation. Seven patients were treated with intra-arterial thrombolysis (one patient failed intra-arterial thrombolysis because of internal carotid artery obstruction), one patient was treated with panretinal photocoagulation, one patient was treated with intravitreal bevacizumab injection, and one patient was treated with combined panretinal photocoagulation and intravitreal bevacizumab injection. Papillomacular bundle involvement was observed in 13 eyes (81.3%) with cilioretinal artery sparing CRAO. Table 3 shows comparative analysis between the good vision group (BCVA ≥20 / 200, n = 3) and the poor vision group (BCVA <20 / 200, n = 13) of patients with cilioretinal artery sparing CRAO according to initial BCVA. BCVA of 20 / 200 was set as the criteria to define good or poor visual outcome groups in reference to prior studies on the visual outcome of eyes with CRAO [9]. In a prior prospective study of non-arteritic CRAO with cilioretinal artery sparing, initial visual acuity was grouped as 20/30 or better in 29% of eyes, 20 / 60 to 20 / 100 in 14% of eyes, 20 / 200 in 6% of eyes, counting fingers in 20% of eyes, and hand motion in 26% of eyes [10]. Mean age (57.33 ± 22.03 vs. 69.23 ± 7.14, p = 0.103), sex ratio (male ratio, 33.3% vs. 61.5%, p = 0.409), initial central macular thickness, and final central macular thickness (357.67 ± 172.02 vs. 354.62 ± 107.84, p = 0.969; 246.67 ± 13.65 vs. 257.85 ± 86.61, p = 0.831) were not significantly different. BCVAs at the initial and final visit were significantly different between the two groups (1.13 ± 1.11 vs 2.25 ± 0.38, p = 0.006; 0.25 ± 0.18 vs 1.98 ± 0.57, p < 0.001). On Goldmann perimetry, all three (100%) patients of the good vision group showed intact central visual fields (within 5 degrees), and only two (15.4%) patients of the poor vision group showed intact central visual fields (p = 0.002). In analysis of the inner retinal structure, features of the papillomacular bundle area on horizontal OCT foveal scans were significantly different in inner retinal thickening and inner retinal hyper-reflectivity between the two groups (1 [33.3%] vs. 12 [92.3%], p = 0.016). Layer-by-layer integrity loss was greater in the poor vision group than in the good vision group, but without statistical significance (1 [33.3%] vs. 9 [69.2%], p = 0.277). Representative images of OCT features and Goldmann perimetry are shown in Fig. 4A–4C, 5A–5D, and 6.
Our study showed that 17.8% (16 / 90) of complete CRAO showed cilioretinal artery sparing CRAO. Visual outcome was generally poor, and only 18.7% of cases showed VA better than 20/200. Good visual outcome was associated with sparing of the whole papillomacular bundle (fovea to disc) from ischemic damage.
Previous studies reported a prevalence of cilioretinal artery sparing CRAO from 14.0% to 26.0% [3]. In normal eyes, the prevalence of one or more cilioretinal arteries has previously been reported to be 49.5% of individuals and 32.1% of eyes [11]. The incidence of cilioretinal artery was 35.0% in all subjects and 18.5% in all eyes in the Han population of north China. Men and women have an equal distribution of cilioretinal arteries [5]. Although there is no research data from Koreans, we infer from this study that about half of cilioretinal arteries showed impaired perfusion in eyes with complete CRAO. This suggests that CRAO is caused by emboli at various locations, such as the central retinal artery only, ophthalmic artery, or multiple branches including the central retinal artery and ciliary arteries.
All cilioretinal arteries ran through the temporal side of the central retinal artery trunk and thus, may possibly perfuse the central fovea. In accordance, initial and final BCVAs and initial central macular thickness were significantly different between the cilioretinal artery sparing group and the non-sparing complete CRAO group. In our previous study, structural changes including initial inner and outer retinal thickening, baseline macula edema, final retinal thinning, and central macular thickness at initial and final presentation differed significantly according to the severity of retinal ischemia or CRAO stages [12]. The degree of macular edema in the acute phase and retinal thinning at final examination correlated significantly with final visual acuity. Therefore, OCT examination may be useful in evaluating retinal ischemic damage and predicting visual prognosis. We previously showed that papillomacular bundle involvement in branch retinal artery occlusion—demonstrated by inner retinal thickening, inner retinal hyper-reflectivity, and loss of layer-by-layer integrity on OCT—could be a meaningful indicator of visual acuity prognosis [8]. In the present study, inner retinal thickening and inner retinal hyper-reflectivity of the papillomacular bundle were significantly different between the good vision group (BCVA ≥20 / 200) and the poor vision group (BCVA <20 / 200) of patients with cilioretinal artery sparing CRAO. These factors could be prognostic markers of visual acuity and visual field defects. Our study confirmed that papillomacular bundle involvement is important for visual prognosis in CRAO, consistent with our previous study on branch retinal artery occlusion [8].
We recently reported five characteristic types of visual field defects that were associated with CRAO stages: peripheral constriction only, paracentral scotoma, central and cecocentral scotoma, temporal island, and no visual field. We also reported that worse stages of CRAO, poor baseline BCVA, thick baseline central macular thickness, and poor baseline OCT morphologic features showed statistically significant associations with baseline severe visual field defects [13]. In the good vision group, all patients showed intact central visual field, namely two central islands and one paracentral scotoma. But in the poor vision group, there was one paracentral scotoma (7.7%), one central and cecocentral scotoma (7.7%), 10 temporal islands (76.9%), and one case with no visual field (7.7%). Papillomacular bundle sparing by cilioretinal artery sparing is associated with central visual field preservation, leading to good visual outcome.
Our study has several limitations including its retrospective nature (possibilities of selection bias) and the small number (n = 16) of patients with cilioretinal artery sparing CRAO. However, there were only several case reports of cilioretinal artery sparing CRAO in the past and there has been no large-scale clinical study. To our knowledge, our study includes the largest number of cases with cilioretinal artery sparing CRAO to date. In that sense, our study is the first report of the incidence of cilioretinal artery sparing CRAO and visual outcomes.
In conclusion, although eyes with cilioretinal artery sparing are common in CRAO and have better prognosis than those with complete CRAO, the visual outcome is generally poor and only a small proportion of cases have preserved small central visual field. Ischemic injury of the papillomacular bundle at the acute stage correlates with poor visual outcome and could be a prognostic factor.
Figures and Tables
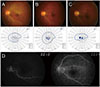 | Fig. 1Clinical manifestation of cilioretinal artery sparing central retinal artery occlusion in one patient (fundus photo, Goldmann perimetry) (A) at initial visit, (B) at 1 month, and (C) at final visit. (D) Initial fundus fluorescein angiography reveals central retinal arterial filling delay and arterio-venous transit time delay except the retinal area which is perfused by the cilioretinal artery (case 8). |
 | Fig. 2Fundus photography (left column) and fluorescein angiographs (right column) of a patient with cilioretinal artery sparing central retinal artery occlusion (A) at initial examination, (B) at 1 day, and (C) after 1 month. Initial and final visual acuities of this patient were hand motion (case 7). |
 | Fig. 3Graphs showing initial and final best-corrected visual acuity (BCVA) between eyes with cilioretinal artery sparing and non-sparing central retinal artery occlusion (CRAO). logMAR = logarithmic minimum angle of resolution. |
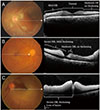 | Fig. 4Representative photographic images of fundus photography and images from horizontal foveal scan of spectral-domain optical coherence tomography (OCT). Lines in fundus photography indicate horizontal OCT scans covering papillomacular bundle area. OCT images show morphologic changes to inner retinal layer of papillomacular bundle area (inner retinal thickening, inner retinal hyper-reflectivity [HR], loss of layer-by-layer integrity). (A) Case 10, (B) case 5, and (C) case 4. |
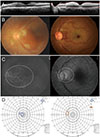 | Fig. 5(A) Optical coherence tomography images showing inner retinal structural changes in papillomacular bundle area and (B) fundus photography, (C) fluorescein angiography, (D) Goldmann perimetry of eye in good vision group (left column, case 10) and eye in poor vision group (right column, case 2). |
 | Fig. 6Visual field features on final Goldmann perimetry of eyes with cilioretinal artery sparing central retinal occlusion. All eyes in good vision group (best-corrected visual acuity ≥20 / 200) showed preserved central visual fields, but most (84.6%) of the poor vision group (best-corrected visual acuity <20 / 200) had impaired central visual fields. |
Table 1
Comparison between demographic and clinical characteristics of cilioretinal artery sparing CRAO and complete CRAO groups

Acknowledgements
This research was partly supported by the Bio & Medical Technology Development Program of the National Research Foundation (NRF) funded by the Korean government (MSIT) (No. 2018M3A9B5021319).
References
1. Varma DD, Cugati S, Lee AW, Chen CS. A review of central retinal artery occlusion: clinical presentation and management. Eye (Lond). 2013; 27:688–697.



2. Cugati S, Varma DD, Chen CS, Lee AW. Treatment options for central retinal artery occlusion. Curr Treat Options Neurol. 2013; 15:63–77.


4. Brown GC, Shields JA. Cilioretinal arteries and retinal arterial occlusion. Arch Ophthalmol. 1979; 97:84–92.


5. Liu L, Liu LM, Chen L. Incidence of cilioretinal arteries in Chinese Han population. Int J Ophthalmol. 2011; 4:323–325.


6. Doguizi S, Sekeroglu MA, Anayol MA, Yilmazbas P. Central retinal artery occlusion with double cilioretinal artery sparing. Retin Cases Brief Rep. 2019; 13:75–78.


7. Bansal N, Bansal RK. A middle hyper-reflective band on spectral domain optical coherence tomography in a case of acute nonarteritic central retinal artery occlusion with sparing of cilioretinal artery. Indian J Ophthalmol. 2019; 67:1345.



8. Cho KH, Ahn SJ, Jung C, et al. Ischemic injury of the papillomacular bundle is a predictive marker of poor vision in eyes with branch retinal artery occlusion. Am J Ophthalmol. 2016; 162:107–120.


9. Chen CS, Lee AW. Management of acute central retinal artery occlusion. Nat Clin Pract Neurol. 2008; 4:376–383.


10. Hayreh SS, Zimmerman MB. Central retinal artery occlusion: visual outcome. Am J Ophthalmol. 2005; 140:376–391.


11. Justice J Jr, Lehmann RP. Cilioretinal arteries. A study based on review of stereo fundus photographs and fluorescein angiographic findings. Arch Ophthalmol. 1976; 94:1355–1358.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download