Abstract
A low-dose chest CT is performed for early detection of lung cancer, but the CT scan frequently shows several incidental abnormalities. Identification of the incidental findings may enable early detection of diseases other than lung cancer, thereby improving the survival of the individual undergoing screening. However, insignificant incidental abnormalities may cause unnecessary additional examination and costs. It is crucial for radiologists to appropriately comprehend and report significant incidental abnormalities other than lung cancer for successful implementation of the national lung cancer screening program in Korea.
Go to : 
References
1. National Lung Screening Trial Research Team. Aberle DR, Adams AM, Berg CD, Black WC, Clapp JD, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening.N Engl J Med. 2011; 365:395–409.
2. National Lung Screening Trial Research Team. Aberle DR, Berg CD, Black WC, Church TR, Fagerstrom RM, et al. The national lung screening trial: overview and study design.Radiology. 2011; 258:243–253.
3. Van de Wiel JC, Wang Y, Xu DM, Van der Zaag-Loonen HJ, Van der Jagt EJ, Van Klaveren RJ, et al. Neglectable benefit of searching for incidental findings in the Dutch-Belgian lung cancer screening trial (NELSON) using low-dose multidetector CT. Eur Radiol. 2007; 17:1474–1482.

4. Jacobs PC, Mali WP, Grobbee DE, Van der Graaf Y. Prevalence of incidental findings in computed tomographic screening of the chest: a systematic review. J Comput Assist Tomogr. 2008; 32:214–221.
5. Lee JW, Kim HY, Goo JM, Kim EY, Lee SJ, Kim TJ, et al. Radiological report of pilot study for the Korean lung cancer screening (K-LUCAS) project: feasibility of implementing lung imaging reporting and data system. Korean J Rad/iiol. 2018; 19:803–808.

6. American College of Radiology. Lung CT screening reporting & data system. Available at.https://www.acr.org/Clinical-Resources/Reporting-and-Data-Systems/Lung-Rads. Published Apr 28, 2014. Accessed Apr 25,. 2019.
7. Kucharczyk MJ, Menezes RJ, McGregor A, Paul NS, Roberts HC. Assessing the impact of incidental findings in a lung cancer screening study by using low-dose computed tomography.Can Assoc Radiol J. 2011; 62:141–145.
8. Detrano R, Guerci AD, Carr JJ, Bild DE, Burke G, Folsom AR, et al. Coronary calcium as a predictor of coronary events in four racial or ethnic groups.N Engl J Med. 2008; 358:1336–1345.
9. Jacobs PC, Gondrie MJ, Van der Graaf Y, De Koning HJ, Isgum I, Van Ginneken B, et al. Coronary artery calcium can predict all-cause mortality and cardiovascular events on low-dose CT screening for lung cancer. AJR Am J Roentgenol. 2012; 198:505–511.

10. Xie X, Zhao Y, De Bock GH, De Jong PA, Mali WP, Oudkerk M, et al. Validation and prognosis of coronary artery calcium scoring in nontriggered thoracic computed tomography: systematic review and metaanalysis. Circ Card/iiovasc Imaging. 2013; 6:514–521.
11. Mets OM, Vliegenthart R, Gondrie MJ, Viergever MA, Oudkerk M, De Koning HJ, et al. Lung cancer screening CT-based prediction of cardiovascular events.JACC Cardiovasc Imaging. 2013; 6:899–907.
12. Chiles C, Duan F, Gladish GW, Ravenel JG, Baginski SG, Snyder BS, et al. Association of coronary artery calcification and mortality in the national lung screening trial: a comparison of three scoring methods.Radiolo-gy. 2015; 276:82–90.
13. Hecht H, Blaha MJ, Berman DS, Nasir K, Budoff M, Leipsic J, et al. Clinical indications for coronary artery calcium scoring in asymptomatic patients: expert consensus statement from the society of cardiovascular computed tomography. J Cardiovasc Comput Tomogr. 2017; 11:157–168.

14. Hecht HS, Cronin P, Blaha MJ, Budoff MJ, Kazerooni EA, Narula J, et al. 2016 SCCT/STR guidelines for coronary artery calcium scoring of noncontrast noncardiac chest CT scans: a report of the society of cardiovascular computed tomography and society of thoracic radiology.J Cardiovasc Comput Tomogr. 2017; 11:74–84.
15. Greenland P, Alpert JS, Beller GA, Benjamin EJ, Budoff MJ, Fayad ZA, et al. 2010 ACCF/AHA guideline for assessment of cardiovascular risk in asymptomatic adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines.J Am Coll Cardiol. 2010; 56:e50–103.
16. Hiratzka LF, Bakris GL, Beckman JA, Bersin RM, Carr VF, Casey DE Jr, et al. 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM guidelines for the diagnosis and management of patients with thoracic aortic disease. A report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines, American Association for Thoracic Surgery, American College of Radiology, American Stroke Association, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of Thoracic Surgeons, and Society for Vascular Medicine. J Am Coll Cardiol. 2010; 55:e27–e129.
18. Hwang YI, Park YB, Yoo KH. Recent trends in the prevalence of chronic obstructive pulmonary disease in Korea. Tuberc Respir Dis (Seoul). 2017; 80:226–229.

19. Durham AL, Adcock IM. The relationship between COPD and lung cancer.Lung Cancer. 2015; 90:121–127.
20. Morgan L, Choi H, Reid M, Khawaja A, Mazzone PJ. Frequency of incidental findings and subsequent evaluation in low-dose computed tomographic scans for lung cancer screening. Ann Am Thorac Soc. 2017; 14:1450–1456.

21. Lynch DA, Austin JH, Hogg JC, Grenier PA, Kauczor HU, Bankier AA, et al. CT-definable subtypes of chronic obstructive pulmonary disease: a statement of the Fleischner society.Radiology. 2015; 277:192–205.
22. Sekine Y, Katsura H, Koh E, Hiroshima K, Fujisawa T. Early detection of COPD is important for lung cancer surveillance.Eur Respir J. 2012; 39:1230–1240.
23. De Torres JP, Bastarrika G, Wisnivesky JP, Alcaide AB, Campo A, Seijo LM, et al. Assessing the relationship between lung cancer risk and emphysema detected on low-dose CT of the chest. Chest. 2007; 132:1932–1938.

24. McWilliams A, Tammemagi MC, Mayo JR, Roberts H, Liu G, Soghrati K, et al. Probability of cancer in pulmonary nodules detected on first screening CT.N Engl J Med. 2013; 369:910–919.
25. Little SA, Sproule MW, Cowan MD, Macleod KJ, Robertson M, Love JG, et al. High resolution computed tomographic assessment of airway wall thickness in chronic asthma: reproducibility and relationship with lung function and severity. Thorax. 2002; 57:247–253.

26. Attili AK, Kazerooni EA, Gross BH, Flaherty KR, Myers JL, Martinez FJ. Smoking-related interstitial lung disease: radiologic-clinical-pathologic correlation. Radiographics. 2008; 28:1383–1396. ;discussion 1396–1398.

27. Jin GY, Lynch D, Chawla A, Garg K, Tammemagi MC, Sahin H, et al. Interstitial lung abnormalities in a CT lung cancer screening population: prevalence and progression rate.Radiology. 2013; 268:563–571.
28. Hunninghake GM. Interstitial lung abnormalities: erecting fences in the path towards advanced pulmonary fibrosis. Thorax. 2019; 74:506–511.

29. Putman RK, Rosas IO, Hunninghake GM. Genetics and early detection in idiopathic pulmonary fibrosis.Am J Respir Crit Care Med. 2014; 189:770–778.
30. Putman RK, Hatabu H, Araki T, Gudmundsson G, Gao W, Nishino M, et al. Association between interstitial lung abnormalities and all-cause mortality.JAMA. 2016; 315:672–681.
31. Dean DS, Gharib H. Epidemiology of thyroid nodules.Best Pract Res Clin Endocrinol Metab. 2008; 22:901–911.
32. MacRedmond R, Logan PM, Lee M, Kenny D, Foley C, Costello RW. Screening for lung cancer using low dose CT scanning. Thorax. 2004; 59:237–241.

33. Nguyen XV, Davies L, Eastwood JD, Hoang JK. Extrapulmonary findings and malignancies in participants screened with chest CT in the national lung screening trial.J Am Coll Radiol. 2017; 14:324–330.
34. Rampinelli C, Preda L, Maniglio M, Sirica L, Travaini LL, Veronesi G, et al. Extrapulmonary malignancies detected at lung cancer screening. Rad/iiology. 2011; 261:293–299.

35. Bahl M, Sosa JA, Nelson RC, Esclamado RM, Choudhury KR, Hoang JK. Trends in incidentally identified thyroid cancers over a decade: a retrospective analysis of 2,090 surgical patients.World J Surg. 2014; 38:1312–1317.
36. Davies L, Welch HG. Current thyroid cancer trends in the United States. JAMA Otolaryngol Head Neck Surg. 2014; 140:317–322.

37. Hoang JK, Langer JE, Middleton WD, Wu CC, Hammers LW, Cronan JJ, et al. Managing incidental thyroid nodules detected on imaging: white paper of the ACR Incidental Thyroid Findings Committee.J Am Coll Radi ol. 2015; 12:143–150.
38. Henschke CI, Lee IJ, Wu N, Farooqi A, Khan A, Yankelevitz D, et al. CT screening for lung cancer: prevalence and incidence of mediastinal masses.Radiology. 2006; 239:586–590.
39. Araki T, Nishino M, Gao W, Dupuis J, Washko GR, Hunninghake GM, et al. Anterior mediastinal masses in the framingham heart study: prevalence and CT image characteristics. Eur J Radiol Open. 2015; 2:26–31.

40. Yoon SH, Choi SH, Kang CH, Goo JM. Incidental anterior mediastinal nodular lesions on chest CT in asymptomatic subjects.J Thorac Oncol. 2018; 13:359–366.
41. Kim H, Yoon SH, Kim J, Lee KW, Choi YR, Cho H, et al. Growth of thymic epithelial tumors and thymic cysts: differential radiological points.Thorac Cancer. 2019; 10:864–871.
42. Carter BW, Okumura M, Detterbeck FC, Marom EM. Approaching the patient with an anterior mediastinal mass: a guide for radiologists.J Thorac Oncol. 2014; 9:S110–S118.
43. Hwang EJ, Paek M, Yoon SH, Kim J, Lee HY, Goo JM, et al. Quantitative thoracic magnetic resonance criteria for the differentiation of cysts from solid masses in the anterior mediastinum. Korean J Radiol. 2019; 20:854–861.

44. Lee SH, Yoon SH, Nam JG, Kim HJ, Ahn SY, Kim HK, et al. Distinguishing between thymic epithelial tumors and benign cysts via computed tomography.Korean J Rad/iiol. 2019; 20:671–682.
45. McKee BJ, Regis SM, McKee AB, Flacke S, Wald C. Performance of ACR lung-RADS in a clinical CT lung screening program.J Am Coll Radiol. 2016; 13:R25–R29.
46. Reiter MJ, Nemesure A, Madu E, Reagan L, Plank A. Frequency and distribution of incidental findings deemed appropriate for S modifier designation on low-dose CT in a lung cancer screening program.Lung Cancer. 2018; 120:1–6.
47. Stigt JA, Boers JE, Oostdijk AH, Van den Berg JW, Groen HJ. Mediastinal incidentalomas. J Thorac Oncol. 2011; 6:1345–1349.

48. Evison M, Crosbie PA, Morris J, Martin J, Barber PV, Booton R. A study of patients with isolated mediastinal and hilar lymphadenopathy undergoing EBUS-TBNA.BMJ Open Respir Res. 2014; 1:e000040.
49. Munden RF, Carter BW, Chiles C, MacMahon H, Black WC, Ko JP, et al. Managing incidental findings on thoracic CT: mediastinal and cardiovascular findings. A white paper of the ACR incidental findings committee.J Am Coll Radiol. 2018; 15:1087–1096.
50. Freedman ND, Abnet CC, Leitzmann MF, Mouw T, Subar AF, Hollenbeck AR, et al. A prospective study of tobacco, alcohol, and the risk of esophageal and gastric cancer subtypes.Am J Epidemiol. 2007; 165:1424–1433.
51. Swensen SJ, Jett JR, Sloan JA, Midthun DE, Hartman TE, Sykes AM, et al. Screening for lung cancer with low-dose spiral computed tomography.Am J Respir Crit Care Med. 2002; 165:508–513.
52. Berland LL, Silverman SG, Gore RM, Mayo-Smith WW, Megibow AJ, Yee J, et al. Managing incidental findings on abdominal CT: white paper of the ACR incidental findings committee.J Am Coll Radiol. 2010; 7:754–773.
53. Mayo-Smith WW, Song JH, Boland GL, Francis IR, Israel GM, Mazzaglia PJ, et al. Management of incidental adrenal masses: a white paper of the ACR incidental findings committee.J Am Coll Radiol. 2017; 14:1038–1044.
54. Mazzone PJ, Silvestri GA, Patel S, Kanne JP, Kinsinger LS, Wiener RS, et al. Screening for lung cancer: CHEST guideline and expert panel report. Chest. 2018; 153:954–985.
Go to : 
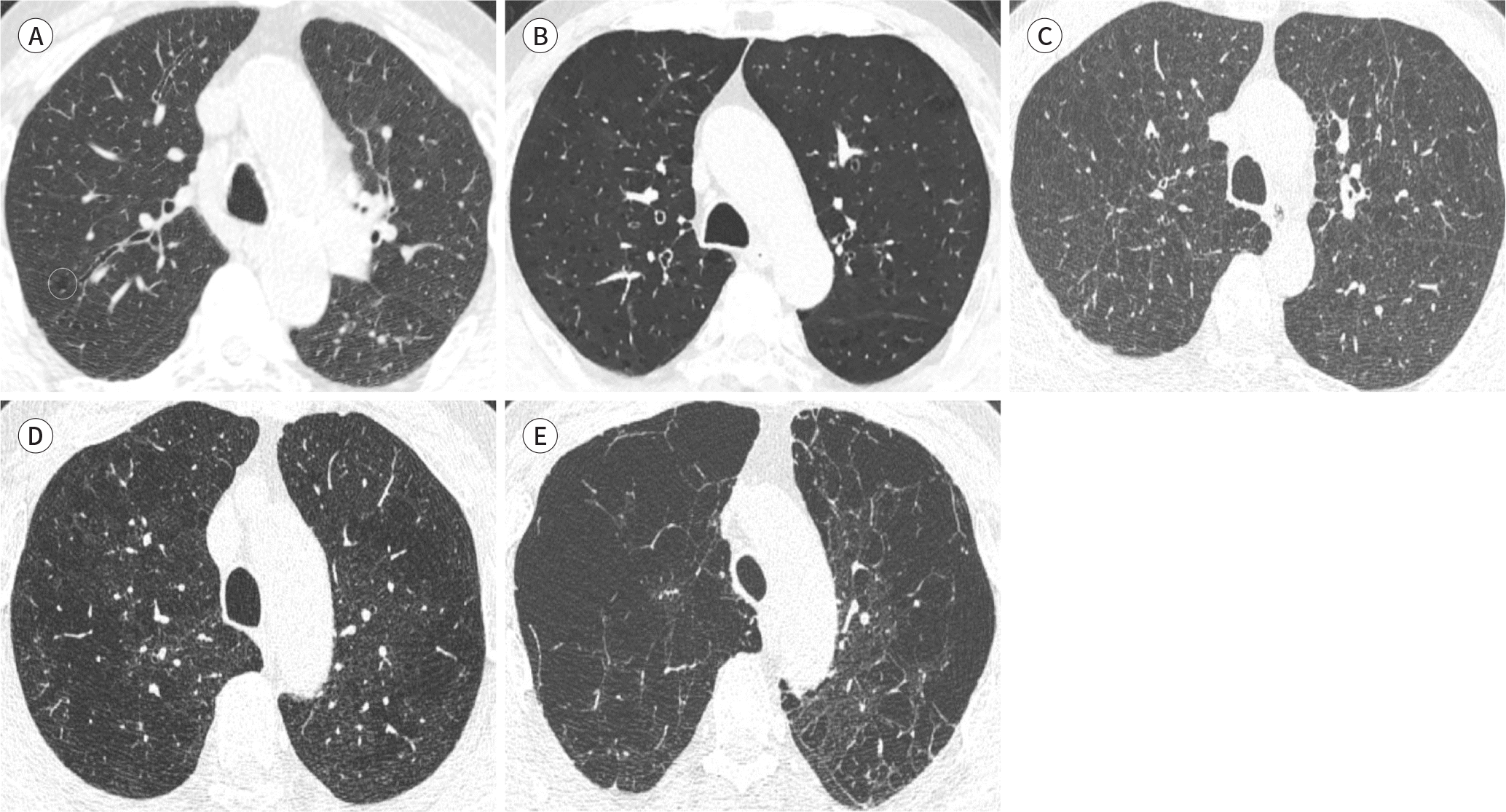 | Fig. 1.Visual grading of emphysema. A. Trace centrilobular emphysema (circle), involving less than 0.5% of the lung zone. B. Mild centrilobular emphysema, involving 0.5–5.0% of the lung zone. C. Moderate centrilobular emphysema, involving over 5% of the lung zone. D. Confluent emphysema. E. Advanced destructive emphysema with vascular distortion. |
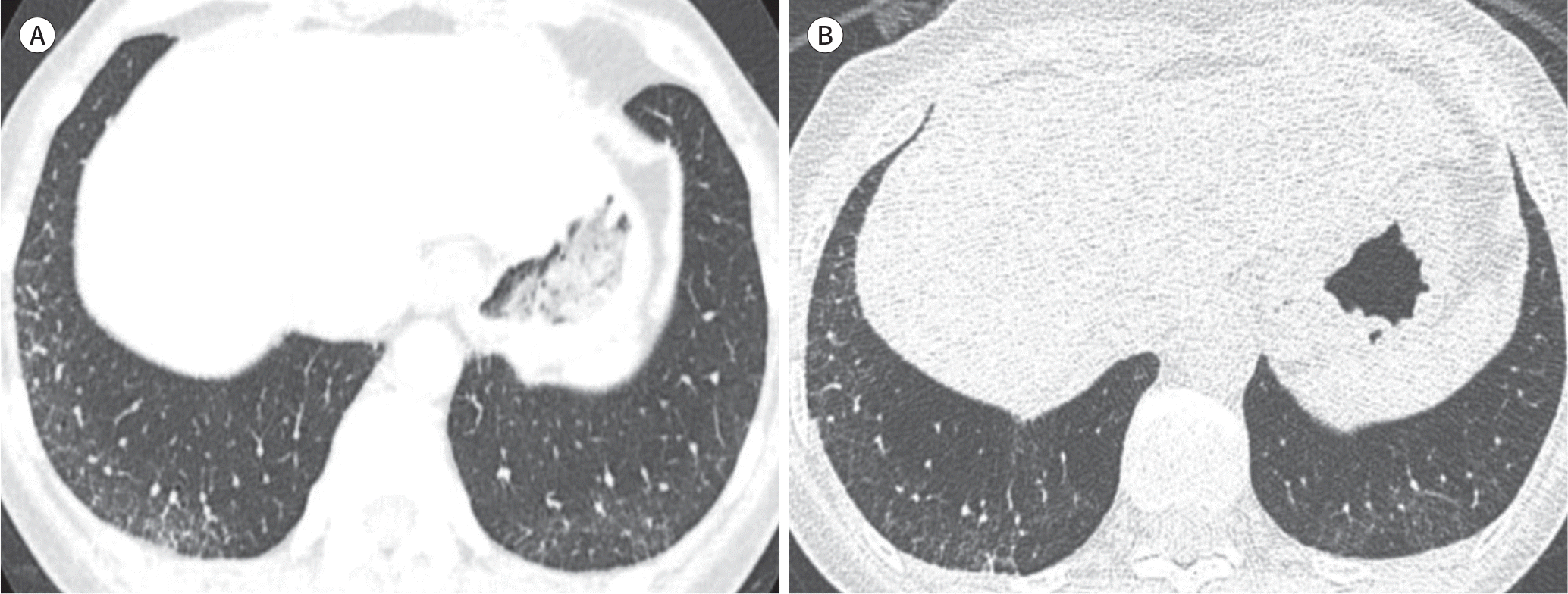 | Fig. 2.Representative images of interstitial lung abnormalities. A. Transverse CT in the supine position shows a mild degree of reticular opacities in the dependent portions of both the lower lobes. B. Subsequently, transverse CT in the prone position confirms that the reticulations and mild ground-glass opacities, rather than gravity-dependent opacities, indicate a mild degree of interstitial abnormality. |
Table 1.
Results of the Delphi Surveys for the Korean Lung Cancer CT Screening
Table 2.
Summary of Significant Abnormalities Other than Lung Cancer in the Korean Lung Cancer CT Screening




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download