Abstract
Cholangiocarcinoma is a disease entity with a wide spectrum of imaging, histological, and clinical features as well as treatment options. At present, imaging studies are essential for the detection, characterization, staging, and resectability assessment of cholangiocarcinoma. This review article describes the imaging features of intrahepatic and perihilar cholangiocarcinoma and the considerations for interpretation of these features. In addition, we introduce the latest concepts regarding the classification system, carcinogenesis process, premalignant lesions, and treatment approaches for cholangiocarcinoma.
Go to : 
References
1. Banales JM, Cardinale V, Carpino G, Marzioni M, Andersen JB, Invernizzi P, et al. Expert consensus document: cholangiocarcinoma: current knowledge and future perspectives consensus statement from the European Network for the Study of Cholangiocarcinoma (ENS-CCA). Nat Rev Gastroenterol Hepatol. 2016; 13:261–280.
2. Zen Y, Adsay NV, Bardadin K, Colombari R, Ferrell L, Haga H, et al. Biliary intraepithelial neoplasia: an international interobserver agreement study and proposal for diagnostic criteria. Mod Pathol. 2007; 20:701–709.

3. Joo I, Lee JM, Yoon JH. Imaging diagnosis of intrahepatic and perihilar cholangiocarcinoma: recent advances and challenges.Radiology. 2018; 288:7–13.
4. Carpino G, Renzi A, Franchitto A, Cardinale V, Onori P, Reid L, et al. Stem/progenitor cell niches involved in hepatic and biliary regeneration.Stem Cells Int. 2016; 2016:3658013.
5. Lanzoni G, Cardinale V, Carpino G. The hepatic, biliary, and pancreatic network of stem/progenitor cell niches in humans: a new reference frame for disease and regeneration.Hepatology. 2016; 64:277–286.
6. Carpino G, Cardinale V, Onori P, Franchitto A, Berloco PB, Rossi M, et al. Biliary tree stem/progenitor cells in glands of extrahepatic and intraheptic bile ducts: an anatomical in situ study yielding evidence of maturational lineages.J Anat. 2012; 220:186–199.
7. Zen Y, Sasaki M, Fujii T, Chen TC, Chen MF, Yeh TS, et al. Different expression patterns of mucin core proteins and cytokeratins during intrahepatic cholangiocarcinogenesis from biliary intraepithelial neoplasia and intraductal papillary neoplasm of the bile duct–an immunohistochemical study of 110 cases of hepatolithiasis. J Hepatol. 2006; 44:350–358.

8. Nakanuma Y, Sasaki M, Sato Y, Ren X, Ikeda H, Harada K. Multistep carcinogenesis of perihilar cholangiocarcinoma arising in the intrahepatic large bile ducts.World J Hepatol. 2009; 1:35–42.
9. Ohtsuka M, Shimizu H, Kato A, Yoshitomi H, Furukawa K, Tsuyuguchi T, et al. Intraductal papillary neoplasms of the bile duct. Int J Hepatol. 2014; 2014:459091.

10. Nakanuma Y, Sudo Y. Biliary tumors with pancreatic counterparts.Semin Diagn Pathol. 2017; 34:167–175.
11. Ohtsuka M, Kimura F, Shimizu H, Yoshidome H, Kato A, Yoshitomi H, et al. Similarities and differences between intraductal papillary tumors of the bile duct with and without macroscopically visible mucin secretion.Am J Surg Pathol. 2011; 35:512–521.
12. Rocha FG, Lee H, Katabi N, DeMatteo RP, Fong Y, D'Angelica MI, et al. Intraductal papillary neoplasm of the bile duct: a biliary equivalent to intraductal papillary mucinous neoplasm of the pancreas?Hepatology. 2012; 56:1352–1360.
13. Bergquist A, Von Seth E. Epidemiology of cholangiocarcinoma.Best Pract Res Clin Gastroenterol. 2015; 29:221–232.
14. Nakeeb A, Pitt HA, Sohn TA, Coleman J, Abrams RA, Piantadosi S, et al. Cholangiocarcinoma. A spectrum of intrahepatic, perihilar, and distal tumors.Ann Surg. 1996; 224:463–473. ; discussion 473–475.

15. Deoliveira ML, Schulick RD, Nimura Y, Rosen C, Gores G, Neuhaus P, et al. New staging system and a registry for perihilar cholangiocarcinoma.Hepatology. 2011; 53:1363–1371.
16. Edge S.American Cancer Society. AJCC cancer staging handbook: from the AJCC cancer staging manual. New York: Springer;2010.
17. Suarez-Munoz MA, Fernandez-Aguilar JL, Sanchez-Perez B, Perez-Daga JA, Garcia-Albiach B, Pulido-Roa Y, et al. Risk factors and classifications of hilar cholangiocarcinoma. World J Gastrointest Oncol. 2013; 5:132–138.

18. Yamasaki S. Intrahepatic cholangiocarcinoma: macroscopic type and stage classification.J Hepatobiliary Pancreat Surg. 2003; 10:288–291.
19. Blechacz B, Komuta M, Roskams T, Gores GJ. Clinical diagnosis and staging of cholangiocarcinoma. Nat Rev Gastroenterol Hepatol. 2011; 8:512–522.

20. Sempoux C, Jibara G, Ward SC, Fan C, Qin L, Roayaie S, et al. Intrahepatic cholangiocarcinoma: new insights in pathology.Semin Liver Dis. 2011; 31:49–60.
22. Kim H, Lim JH, Jang KT, Kim MJ, Lee J, Lee JY, et al. Morphology of intraductal papillary neoplasm of the bile ducts: radiologic-pathologic correlation. Abdom Imaging. 2011; 36:438–446.

23. Sakamoto E, Nimura Y, Hayakawa N, Kamiya J, Kondo S, Nagino M, et al. The pattern of infiltration at the proximal border of hilar bile duct carcinoma: a histologic analysis of 62 resected cases.Ann Surg. 1998; 227:405–411.
24. Akamatsu N, Sugawara Y, Hashimoto D. Surgical strategy for bile duct cancer: advances and current limitations. World J Clin Oncol. 2011; 2:94–107.

25. Okuda K, Kubo Y, Okazaki N, Arishima T, Hashimoto M. Clinical aspects of intrahepatic bile duct carcinoma including hilar carcinoma: a study of 57 autopsy-proven cases. Cancer. 1977; 39:232–246.
26. Nakanuma Y, Sato Y, Harada K, Sasaki M, Xu J, Ikeda H. Pathological classification of intrahepatic cholangiocarcinoma based on a new concept.World J Hepatol. 2010; 2:419–427.
27. Komuta M, Govaere O, Vandecaveye V, Akiba J, Van Steenbergen W, Verslype C, et al. Histological diversity in cholangiocellular carcinoma reflects the different cholangiocyte phenotypes.Hepatology. 2012; 55:1876–1888.
28. Cardinale V, Carpino G, Reid L, Gaudio E, Alvaro D. Multiple cells of origin in cholangiocarcinoma underlie biological, epidemiological and clinical heterogeneity.World J Gastrointest Oncol. 2012; 4:94–102.
29. Razumilava N, Gores GJ. Classification, diagnosis, and management of cholangiocarcinoma.Clin Gastroenterol Hepatol. 2013; 11:13–21. .e1; quiz e3–4.
30. Ruys AT, Van Beem BE, Engelbrecht MR, Bipat S, Stoker J, Van Gulik TM. Radiological staging in patients with hilar cholangiocarcinoma: a systematic review and metaanalysis. Br J Radiol. 2012; 85:1255–1262.

31. Joo I, Lee JM. Imaging bile duct tumors: pathologic concepts, classification, and early tumor detection. Ab-d om Imaging. 2013; 38:1334–1350.

32. Lee HY, Kim SH, Lee JM, Kim SW, Jang JY, Han JK, et al. Preoperative assessment of resectability of hepatic hilar cholangiocarcinoma: combined CT and cholangiography with revised criteria.Radiology. 2006; 239:113–121.
34. Zandrino F, Benzi L, Ferretti ML, Ferrando R, Reggiani G, Musante F. Multislice CT cholangiography without biliary contrast agent: technique and initial clinical results in the assessment of patients with biliary obstruction. Eur Radiol. 2002; 12:1155–1161.

35. Ahmetoglu A, Kos¸ucu P, Kul S, Dinç H, Sari A, Arslan M, et al. MDCT cholangiography with volume rendering for the assessment of patients with biliary obstruction. AJR Am J Roentgenol. 2004; 183:1327–1332.
36. Ryoo I, Lee JM, Park HS, Han JK, Choi BI. Preoperative assessment of longitudinal extent of bile duct cancers using MDCT with multiplanar reconstruction and minimum intensity projections: comparison with MR cholangiography. Eur J Radiol. 2012; 81:2020–2026.

37. Jhaveri KS, Hosseini-Nik H. MRI of cholangiocarcinoma. J Magn Reson Imaging. 2015; 42:1165–1179.

38. Anupindi SA, Victoria T. Magnetic resonance cholangiopancreatography: techniques and applications. Magn Reson Imaging Clin N Am. 2008; 16:453–466. v.

39. Chandarana H, Doshi AM, Shanbhogue A, Babb JS, Bruno MT, Zhao T, et al. Three-dimensional MR cholangiopancreatography in a breath hold with sparsity-based reconstruction of highly undersampled data.Radiology. 2016; 280:585–594.
40. Yoon JH, Lee SM, Kang HJ, Weiland E, Raithel E, Son Y, et al. Clinical feasibility of 3-dimensional magnetic resonance cholangiopancreatography using compressed sensing: comparison of image quality and diagnostic performance. Invest Radiol. 2017; 52:612–619.
41. Masselli G, Manfredi R, Vecchioli A, Gualdi G. MR imaging and MR cholangiopancreatography in the preoperative evaluation of hilar cholangiocarcinoma: correlation with surgical and pathologic findings.Eur Radiol. 2008; 18:2213–2221.
42. Gschwend S, Ebert W, Schultze-Mosgau M, Breuer J. Pharmacokinetics and imaging properties of Gd-EOB-DTPA in patients with hepatic and renal impairment.Invest Radiol. 2011; 46:556–566.
43. Kim R, Lee JM, Shin CI, Lee ES, Yoon JH, Joo I, et al. Differentiation of intrahepatic mass-forming cholangiocarcinoma from hepatocellular carcinoma on gadoxetic acid-enhanced liver MR imaging.Eur Radiol. 2016; 26:1808–1817.
44. Matos C, Serrao E, Bali MA. Magnetic resonance imaging of biliary tumors.Magn Reson Imaging Clin N Am. 2010; 18:477–496. x.
45. Kim HJ, Lee SS, Byun JH, Kim JC, Yu CS, Park SH, et al. Incremental value of liver MR imaging in patients with potentially curable colorectal hepatic metastasis detected at CT: a prospective comparison of diffusion-weighted imaging, gadoxetic acid-enhanced MR imaging, and a combination of both MR techniques. Radi-ology. 2015; 274:712–722.

46. Kim JY, Kim MH, Lee TY, Hwang CY, Kim JS, Yun SC, et al. Clinical role of 18F-FDG PET-CT in suspected and potentially operable cholangiocarcinoma: a prospective study compared with conventional imaging.Am J Gastroenterol. 2008; 103:1145–1151.
47. Aljiffry M, Abdulelah A, Walsh M, Peltekian K, Alwayn I, Molinari M. Evidence-based approach to cholangiocarcinoma: a systematic review of the current literature.J Am Coll Surg. 2009; 208:134–147.
48. Chung YE, Kim MJ, Park YN, Choi JY, Pyo JY, Kim YC, et al. Varying appearances of cholangiocarcinoma: radiologic-pathologic correlation. Radiographics. 2009; 29:683–700.

49. Kang Y, Lee JM, Kim SH, Han JK, Choi BI. Intrahepatic mass-forming cholangiocarcinoma: enhancement patterns on gadoxetic acid-enhanced MR images. Rad/iiology. 2012; 264:751–760.

50. Shah A, Tang A, Santillan C, Sirlin C. Cirrhotic liver: what's that nodule? The LI-RADS approach.J Magn Reson Imaging. 2016; 43:281–294.
51. Park HJ, Kim YK, Park MJ, Lee WJ. Small intrahepatic mass-forming cholangiocarcinoma: target sign on diffusion-weighted imaging for differentiation from hepatocellular carcinoma. Abdom Imaging. 2013; 38:793–801.
52. Jeong HT, Kim MJ, Kim YE, Park YN, Choi GH, Choi JS. MRI features of hepatocellular carcinoma expressing progenitor cell markers. Liver Int. 2012; 32:430–440.

53. Kim SA, Lee JM, Lee KB, Kim SH, Yoon SH, Han JK, et al. Intrahepatic mass-forming cholangiocarcinomas: enhancement patterns at multiphasic CT, with special emphasis on arterial enhancement pattern–correlation with clinicopathologic findings.Radiology. 2011; 260:148–157.
54. Huang B, Wu L, Lu XY, Xu F, Liu CF, Shen WF, et al. Small intrahepatic cholangiocarcinoma and hepatocellular carcinoma in cirrhotic livers may share similar enhancement patterns at multiphase dynamic MR imaging. Radiology. 2016; 281:150–157.

55. Joo I, Lee JM, Lee SM, Lee JS, Park JY, Han JK. Diagnostic accuracy of liver imaging reporting and data system (LI-RADS) v2014 for intrahepatic mass-forming cholangiocarcinomas in patients with chronic liver disease on gadoxetic acid-enhanced MRI. J Magn Reson Imaging. 2016; 44:1330–1338.

56. Rimola J, Forner A, Reig M, Vilana R, De Lope CR, Ayuso C, et al. Cholangiocarcinoma in cirrhosis: absence of contrast washout in delayed phases by magnetic resonance imaging avoids misdiagnosis of hepatocellular carcinoma.Hepatology. 2009; 50:791–798.
57. Joo I, Lee JM, Lee DH, Jeon JH, Han JK. Retrospective validation of a new diagnostic criterion for hepatocellular carcinoma on gadoxetic acid-enhanced MRI: can hypointensity on the hepatobiliary phase be used as an alternative to washout with the aid of ancillary features?Eur Radiol. 2019; 29:1724–1732.
58. Kamath A, Roudenko A, Hecht E, Sirlin C, Chernyak V, Fowler K, et al. CT/MR LI-RADS 2018: clinical implications and management recommendations. Abdom Radiol (NY). 2019; 44:1306–1322.

59. Türkoglu MA, Yamamoto Y, Sugiura T, Okamura Y, Ito T, Ashida R, et al. The favorable prognosis after operative resection of hypervascular intrahepatic cholangiocarcinoma: a clinicopathologic and immunohistochemical study.Surgery. 2016; 160:683–690.
60. Ariizumi S, Kotera Y, Takahashi Y, Katagiri S, Chen IP, Ota T, et al. Mass-forming intrahepatic cholangiocarcinoma with marked enhancement on arterial-phase computed tomography reflects favorable surgical outcomes. J Surg Oncol. 2011; 104:130–139.

61. Lee J, Kim SH, Kang TW, Song KD, Choi D, Jang KT. Mass-forming intrahepatic cholangiocarcinoma: diffusion-weighted imaging as a preoperative prognostic marker. Radiology. 2016; 281:119–128.

62. Asayama Y, Yoshimitsu K, Irie H, Tajima T, Nishie A, Hirakawa M, et al. Delayed-phase dynamic CT enhancement as a prognostic factor for mass-forming intrahepatic cholangiocarcinoma.Radiology. 2006; 238:150–155.
63. Koh J, Chung YE, Nahm JH, Kim HY, Kim KS, Park YN, et al. Intrahepatic mass-forming cholangiocarcinoma: prognostic value of preoperative gadoxetic acid-enhanced MRI.Eur Radiol. 2016; 26:407–416.
64. Kajiyama K, Maeda T, Takenaka K, Sugimachi K, Tsuneyoshi M. The significance of stromal desmoplasia in intrahepatic cholangiocarcinoma: a special reference of ‘scirrhous-type'and ‘nonscirrhous-type'growth.Am J Surg Pathol. 1999; 23:892–902.
65. Rizvi S, Gores GJ. Pathogenesis, diagnosis, and management of cholangiocarcinoma.Gastroenterology. 2013; 145:1215–1229.
66. Fujita N, Asayama Y, Nishie A, Ishigami K, Ushijima Y, Takayama Y, et al. Mass-forming intrahepatic cholangiocarcinoma: enhancement patterns in the arterial phase of dynamic hepatic CT-Correlation with clinicopathological findings. Eur Radiol. 2017; 27:498–506.
67. Valls C, Ruiz S, Martinez L, Leiva D. Radiological diagnosis and staging of hilar cholangiocarcinoma.World J Gastrointest Oncol. 2013; 5:115–126.
68. Kim JY, Lee JM, Han JK, Kim SH, Lee JY, Choi JY, et al. Contrast-enhanced MRI combined with MR cholangiopancreatography for the evaluation of patients with biliary strictures: differentiation of malignant from benign bile duct strictures.J Magn Reson Imaging. 2007; 26:304–312.
69. Choi SH, Han JK, Lee JM, Lee KH, Kim SH, Lee JY, et al. Differentiating malignant from benign common bile duct stricture with multiphasic helical CT.Radiology. 2005; 236:178–183.
70. Yoo RE, Lee JM, Yoon JH, Kim JH, Han JK, Choi BI. Differential diagnosis of benign and malignant distal biliary strictures: value of adding diffusion-weighted imaging to conventional magnetic resonance cholangiopancreatography. J Magn Reson Imaging. 2014; 39:1509–1517.

71. Choi KS, Lee JM, Joo I, Han JK, Choi BI. Evaluation of perihilar biliary strictures: does DWI provide additional value to conventional MRI?AJR Am J Roentgenol. 2015; 205:789–796.
72. Park TG, Yu YD, Park BJ, Cheon GJ, Oh SY, Kim DS, et al. Implication of lymph node metastasis detected on 18F-FDG PET/CT for surgical planning in patients with peripheral intrahepatic cholangiocarcinoma. Clin Nucl Med. 2014; 39:1–7.

73. Furukawa H, Ikuma H, Asakura-Yokoe K, Uesaka K. Preoperative staging of biliary carcinoma using 18F-fluoro-deoxyglucose PET: prospective comparison with PET+CT, MDCT and histopathology.Eur Radiol. 2008; 18:2841–2847.
74. Anderson CD, Rice MH, Pinson CW, Chapman WC, Chari RS, Delbeke D. Fluorodeoxyglucose PET imaging in the evaluation of gallbladder carcinoma and cholangiocarcinoma. J Gastrointest Surg. 2004; 8:90–97.

75. Petrowsky H, Wildbrett P, Husarik DB, Hany TF, Tam S, Jochum W, et al. Impact of integrated positron emission tomography and computed tomography on staging and management of gallbladder cancer and cholangiocarcinoma. J Hepatol. 2006; 45:43–50.

76. Fritscher-Ravens A, Bohuslavizki KH, Broering DC, Jenicke L, Schäfer H, Buchert R, et al. FDG PET in the diagnosis of hilar cholangiocarcinoma. Nucl Med Commun. 2001; 22:1277–1285.

77. Domagk D, Poremba C, Dietl KH, Senninger N, Heinecke A, Domschke W, et al. Endoscopic transpapillary biopsies and intraductal ultrasonography in the diagnostics of bile duct strictures: a prospective study.Gut. 2002; 51:240–244.
78. Naitoh I, Nakazawa T, Kato A, Hayashi K, Miyabe K, Shimizu S, et al. Predictive factors for positive diagnosis of malignant biliary strictures by transpapillary brush cytology and forceps biopsy. J Dig Dis. 2016; 17:44–51.

79. De Bellis M, Sherman S, Fogel EL, Cramer H, Chappo J, McHenry L Jr, et al. Tissue sampling at ERCP in suspected malignant biliary strictures (Part 2). Gastrointest Endosc. 2002; 56:720–730.

80. Ryoo I, Lee JM, Chung YE, Park HS, Kim SH, Han JK, et al. Gadobutrol-enhanced, three-dimensional, dynamic MR imaging with MR cholangiography for the preoperative evaluation of bile duct cancer.Invest Radiol. 2010; 45:217–224.
81. Sun HY, Lee JM, Park HS, Yoon JH, Baek JH, Han JK, et al. Gadoxetic acid-enhanced MRI with MR cholangiography for the preoperative evaluation of bile duct cancer.J Magn Reson Imaging. 2013; 38:138–147.
82. Han IW, Jang JY, Kang MJ, Kwon W, Park JW, Chang YR, et al. Role of resection for Bismuth type IV hilar cholangiocarcinoma and analysis of determining factors for curative resection. Ann Surg Treat Res. 2014; 87:87–93.

83. Ribero D, Zimmitti G, Aloia TA, Shindoh J, Forchino F, Amisano M, et al. Preoperative cholangitis and future liver remnant volume determine the risk of liver failure in patients undergoing resection for hilar cholangiocarcinoma. J Am Coll Surg. 2016; 223:87–97.

84. Gotra A, Sivakumaran L, Chartrand G, Vu KN, Vandenbroucke-Menu F, Kauffmann C, et al. Liver segmentation: indications, techniques and future directions. Insights Imaging. 2017; 8:377–392.

85. Sato Y, Matsushima S, Inaba Y, Sano T, Yamaura H, Kato M, et al. Preoperative estimation of future remnant liver function following portal vein embolization using relative enhancement on gadoxetic acid disodium-enhanced magnetic resonance imaging. Korean J Radiol. 2015; 16:523–530.

86. Yoon JH, Choi JI, Jeong YY, Schenk A, Chen L, Laue H, et al. Pre-treatment estimation of future remnant liver function using gadoxetic acid MRI in patients with HCC. J Hepatol. 2016; 65:1155–1162.

87. Lee DH, Lee JM, Yi NJ, Lee KW, Suh KS, Lee JH, et al. Hepatic stiffness measurement by using MR elastography: prognostic values after hepatic resection for hepatocellular carcinoma.Eur Radiol. 2017; 27:1713–1721.
88. Cescon M, Colecchia A, Cucchetti A, Peri E, Montrone L, Ercolani G, et al. Value of transient elastography measured with FibroScan in predicting the outcome of hepatic resection for hepatocellular carcinoma.Ann Surg. 2012; 256:706–712. ; discussion 712–713.
89. Olthof PB, Wiggers JK, Groot Koerkamp B, Coelen RJ, Allen PJ, Besselink MG, et al. Postoperative liver failure risk score: identifying patients with resectable perihilar cholangiocarcinoma who can benefit from portal vein embolization.J Am Coll Surg. 2017; 225:387–394.
90. Wiggers JK, Groot Koerkamp B, Cieslak KP, Doussot A, Van Klaveren D, Allen PJ, et al. Postoperative mortality after liver resection for perihilar cholangiocarcinoma: development of a risk score and importance of biliary drainage of the future liver remnant.J Am Coll Surg. 2016; 223:321–331. .e1.
91. Corpechot C, Gaouar F, El Naggar A, Kemgang A, Wendum D, Poupon R, et al. Baseline values and changes in liver stiffness measured by transient elastography are associated with severity of fibrosis and outcomes of patients with primary sclerosing cholangitis. Gastroenterology. 2014; 146:970–979. ; quiz e15-e16.

92. Higuchi R, Yamamoto M. Indications for portal vein embolization in perihilar cholangiocarcinoma.J Hepatobiliary Pancreat Sci. 2014; 21:542–549.
93. Lim JH, Yoon KH, Kim SH, Kim HY, Lim HK, Song SY, et al. Intraductal papillary mucinous tumor of the bile ducts. Radiograph/iics. 2004; 24:53–66. ; discussion 66–67.

94. Wan XS, Xu YY, Qian JY, Yang XB, Wang AQ, He L, et al. Intraductal papillary neoplasm of the bile duct. World J Gastroenterol. 2013; 19:8595–8604.

95. Lim JH, Zen Y, Jang KT, Kim YK, Nakanuma Y. Cyst-forming intraductal papillary neoplasm of the bile ducts: description of imaging and pathologic aspects. AJR Am J Roentgenol. 2011; 197:1111–1120.

96. Hong GS, Byun JH, Kim JH, Kim HJ, Lee SS, Hong SM, et al. Thread sign in biliary intraductal papillary mucinous neoplasm: a novel specific finding for MRI. Eur Radiol. 2016; 26:3112–3120.

97. Zen Y, Pedica F, Patcha VR, Capelli P, Zamboni G, Casaril A, et al. Mucinous cystic neoplasms of the liver: a clinicopathological study and comparison with intraductal papillary neoplasms of the bile duct.Mod Pathol. 2011; 24:1079–1089.
98. Kang MJ, Jang JY, Lee KB, Han IW, Kim SW. Impact of macroscopic morphology, multifocality, and mucin secretion on survival outcome of intraductal papillary neoplasm of the bile duct.J Gastrointest Surg. 2013; 17:931–938.
99. Jung G, Park KM, Lee SS, Yu E, Hong SM, Kim J. Long-term clinical outcome of the surgically resected intraductal papillary neoplasm of the bile duct.J Hepatol. 2012; 57:787–793.
Go to : 
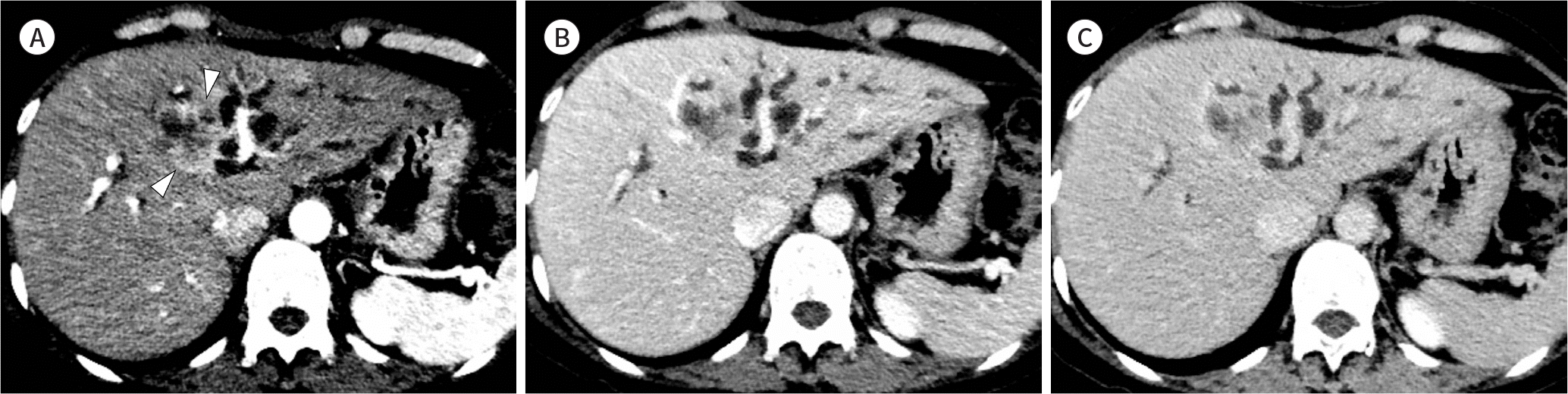 | Fig. 1.Images from a 48-year-old woman with intrahepatic mass-forming type cholangiocarcinoma. A-C. Axial CT images show a solid mass in segment 4 of the liver. The mass demonstrates peripheral enhancement (arrowheads) in the arterial phase (A) and progressive centripetal enhancement in the portal-venous phase (B) and delayed phase (C). Note the dilated intrahepatic ducts peripheral to the mass. |
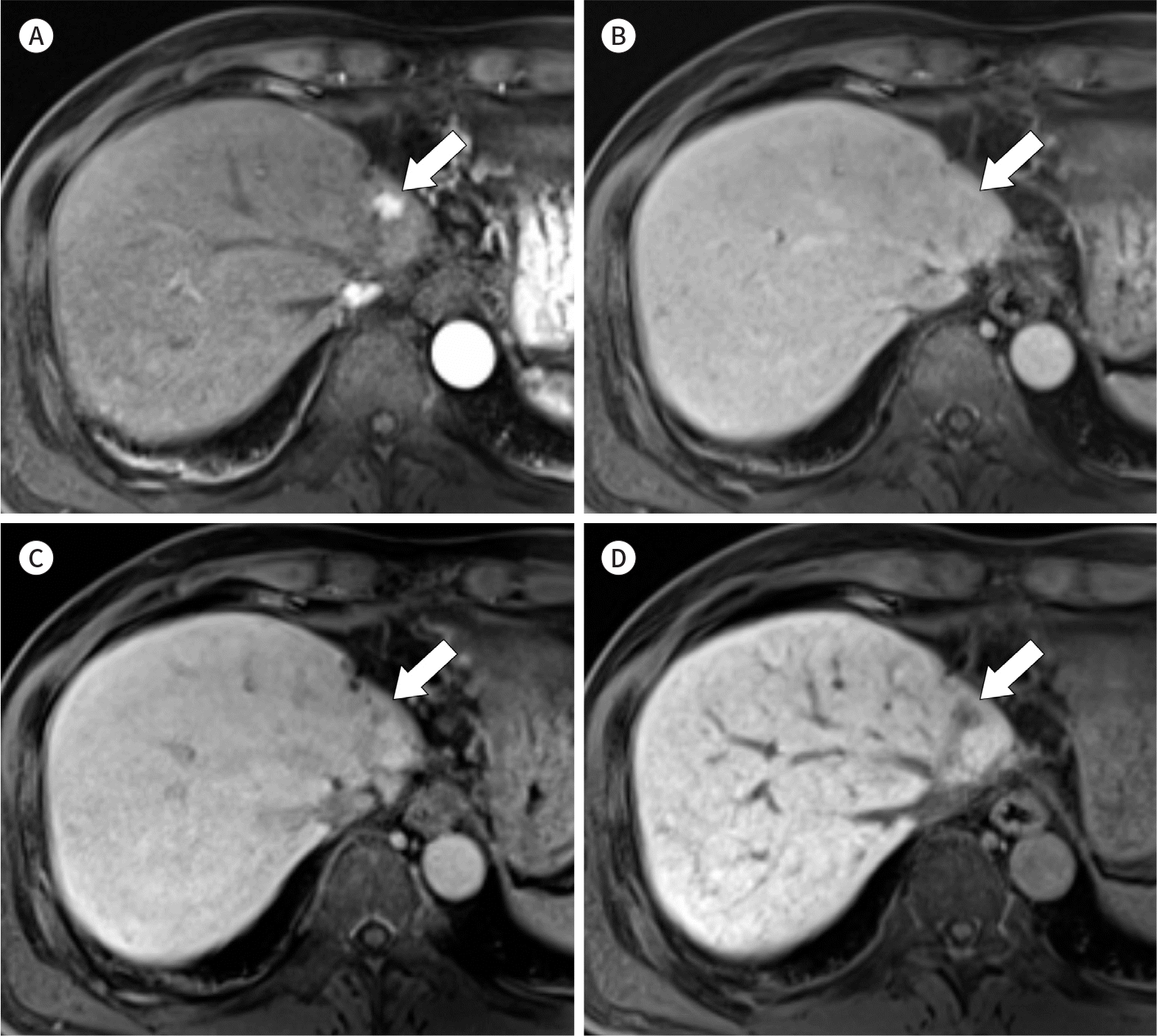 | Fig. 2.Images from a 60-year-old man with intrahepatic mass-forming type cholangiocarcinoma and underlying chronic hepatitis B. This patient had previously undergone hepatic resection for hepatocellular carcinoma. A-D. On gadoxetic acid-enhanced MRI, a 1-cm nodular lesion (arrows) is seen adjacent to the margin of hepatic resection. The mass shows nodular hyper-enhancement (not-rim) in the arterial phase (A) and lacks washout in the portal venous phase (B). During the transitional phase (C) and hepatobiliary phase (D), the nodule shows hypointensity relative to the liver parenchyma. Surgical resection and histology confirmed the diagnosis of cholangiocarcinoma. |
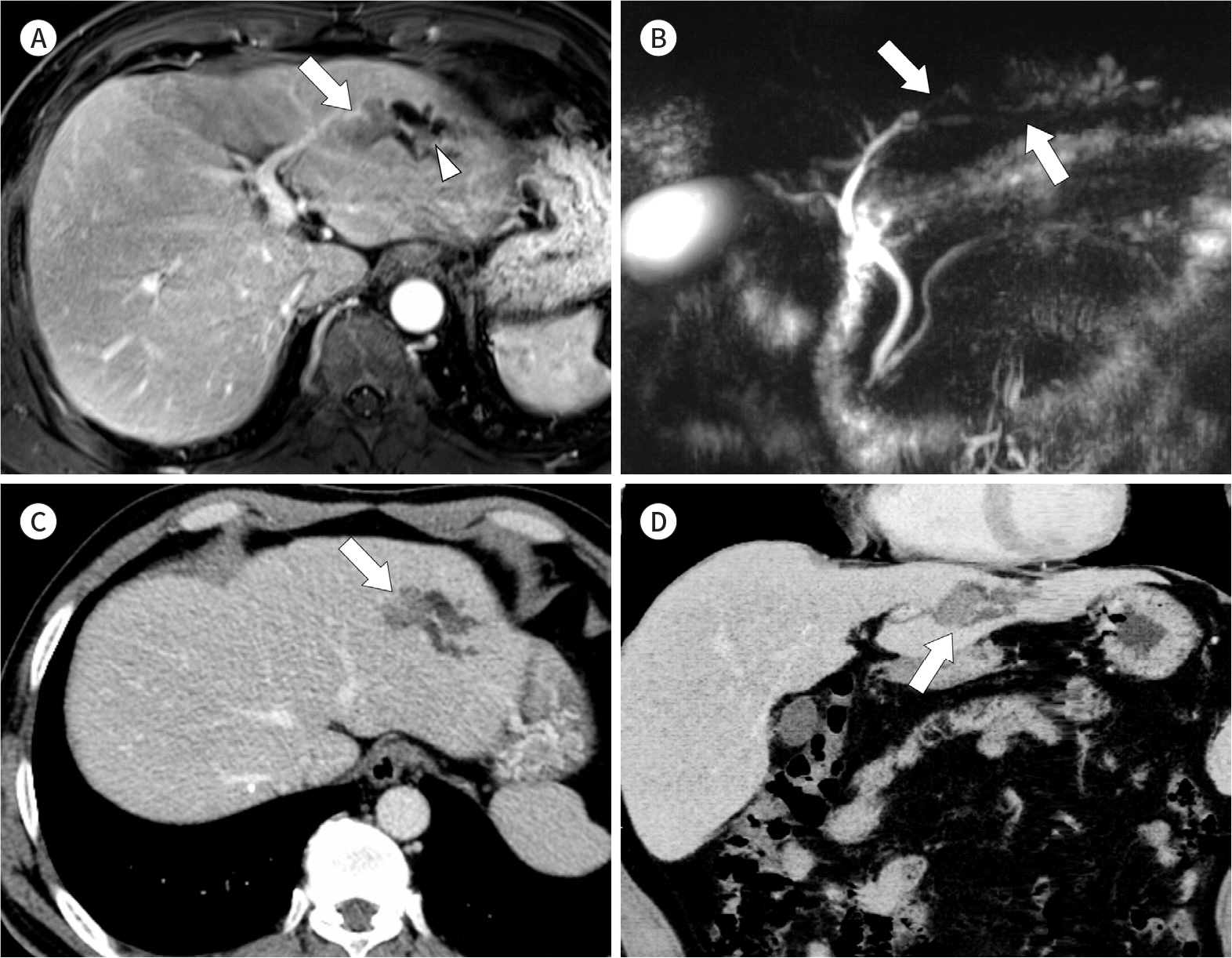 | Fig. 3.Images from a 54-year-old man with intrahepatic, intraductal-growing type cholangiocarcinoma. A. On the portal venous phase image using contrast-enhanced T1-weighted MRI, an intraductal tumor (arrow) is seen in B3. The affected segment of the bile duct and upstream duct are dilated. Another small intraductal tumor (arrowhead), or skip lesion, is also identified. B. Three-dimensional MR cholangiopancreatography shows segmental filing defects (arrows) in the intrahepatic duct. C. A contrast-enhanced CT image shows the intraductal tumor (arrow) with a mild degree of enhancement. D. CT cholangiography using minimum intensity projection and curved multiplanar reconstruction demonstrates the intraductal |
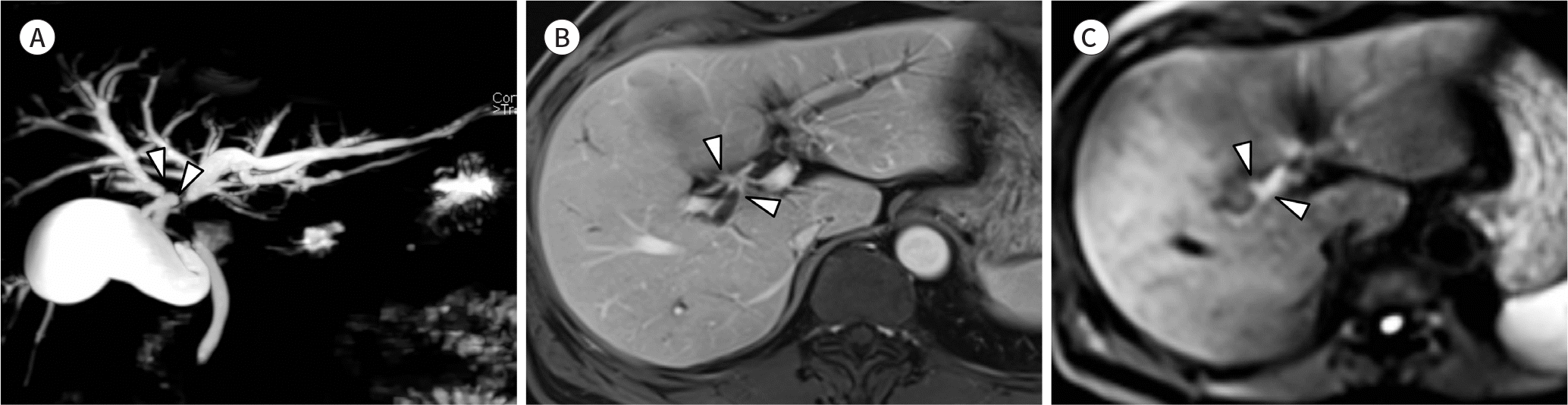 | Fig. 4.Images from a 57-year-old man with periductal infiltrating type perihilar cholangiocarcinoma. This patient underwent an extended right hemihepatectomy with curative intent. However, there was microscopic tumor involvement in the proximal margin of resection (left hepatic duct). A. Three-dimensional MR cholangiopancreatography shows dilatation of bilateral intrahepatic ducts and separation of the right anterior and posterior ducts (arrowheads) while the left second confluence is preserved, suggesting Bismuth-Corlette classification type IIIA. B. The axial portal venous phase image with contrast-enhanced T1-weighted MRI shows thickening of the hilar bile ducts with enhancement. Separation of right anterior and posterior ducts is seen (arrowheads). C. On the diffusion-weighted image obtained with a b value of 800 sec/mm2, the perihilar cholangiocarcinoma shows restricted diffusion. The area of restricted diffusion along the bile duct is almost identical to that of the enhanced wall thickening found on contrast-enhanced T1-weighted MRI (arrowheads). |
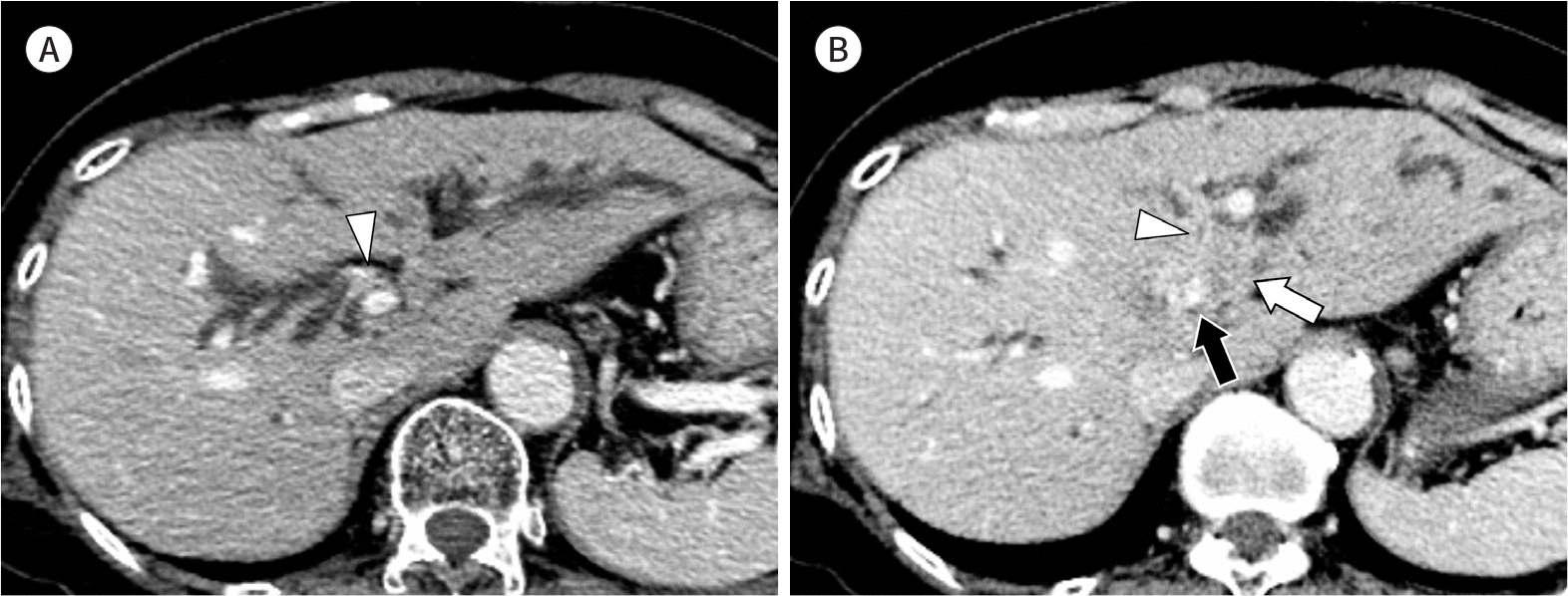 | Fig. 5.Images from a 61-year-old woman with perihilar cholangiocarcinoma of mixed mass-forming and periductal infiltrating type. A, B. Axial portal venous phase CT images show enhancing wall thickening of both the right hepatic duct (arrowhead, A) and the left hepatic duct (arrowhead, B). B. An intrahepatic mass (white arrow) connected to the perihilar tumor is seen in the left lobe of the liver. Invasion of the tumor has caused segmental narrowing of the proximal left portal vein (black arrow). |
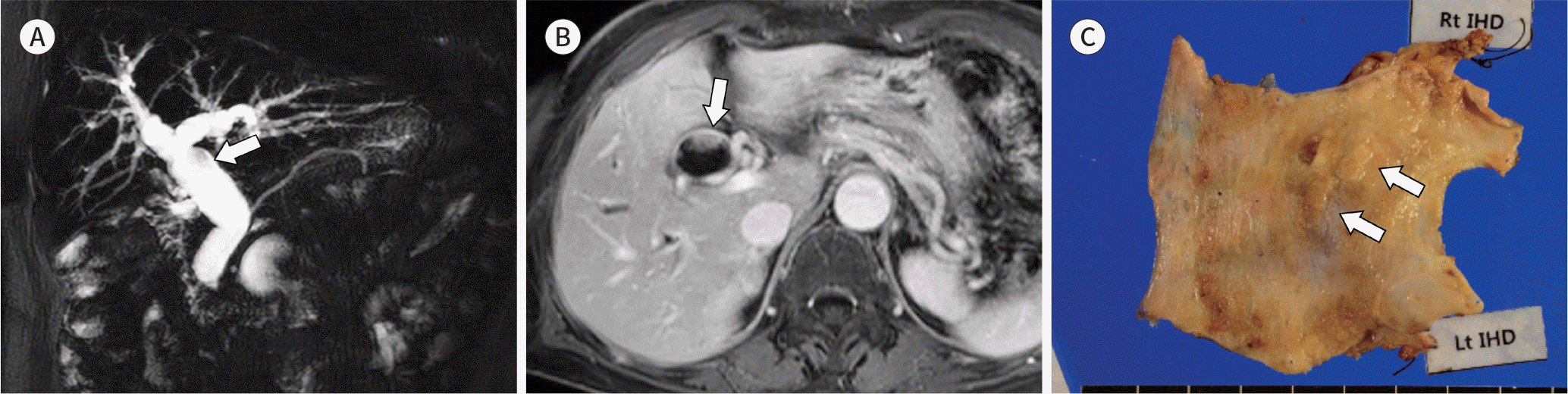 | Fig. 6.Images from a 90-year-old woman with mucin-hypersecreting intraductal papillary neoplasm of the bile duct. A. Two-dimensional MR cholangiopancreatography shows fusiform dilatation of the extrahepatic duct. A small filling defect (arrow) is seen in the common hepatic duct due to a solid intraductal tumor. Both the upstream and downstream bile ducts are dilated. B. Axial contrast-enhanced T1-weighted MRI demonstrates an enhancing intraductal tumor (arrow). C. A photograph of the gross specimen shows intraductal papillary tumors (arrows) in the common hepatic duct. |
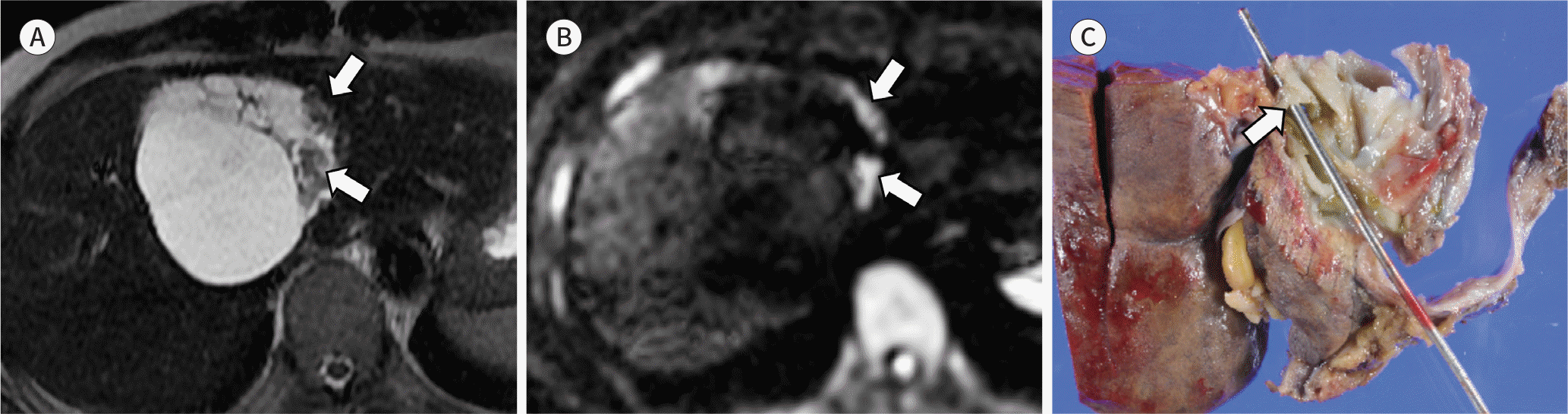 | Fig. 7.Images from a 42-year-old man with a surgically confirmed cyst-forming IPNB. A. An axial heavily T2-weighted image shows a lobulated cystic mass in segment 4 of the liver with internal septa and mural nodules (arrows). The intrahepatic bile ducts are not dilated. B. On a diffusion-weighted image (b value = 1000 sec/mm2), the mural nodules show restricted diffusion (i.e. high signal intensity) (arrows). C. On gross specimen, the communication between the bile duct and cystic mass is identifiable (arrow). Histological examination confirms the diagnosis of IPNB with high-grade intraepithelial neoplasia. IPNB = intraductal papillary neoplasm of the bile duct. |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download