Abstract
Automated breast ultrasonography (ABUS) is a recently introduced technology. Similar to handheld ultrasound (HHUS), it is a supplementary screening test for breast cancer to be used along with mammography. It is particularly useful for detecting invasive breast cancers that may be overlooked by mammography in denser breast tissue. The use of ABUS is becomingmore common because of the advantages of low operator dependency during image acquisition, high reproducibility, a wide field-of-view, and unavailability of better coronal imaging with HHUS. Consequently, there have been suggestions to extend ABUS use to diagnostic screening. Therefore, in this paper, we provide a review of the literature and discuss the usefulness and value of ABUS in breast cancer screening.
Go to : 
References
1. Bernard W, Christopher PW. World cancer report 2014. International agency for research on cancer. Lyon: World Health Organization;2014.
2. International Agency for Research on Cancer. GLOBOCAN 2012: estimated cancer incidence, mortality and prevalence worldwide. Available at.http://gco.iarc.fr. Accessed Aug 14,. 2018.
3. Jung KW, Won YJ, Kong HJ, Lee ES. Community of Population-Based Regional Cancer Registries. Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2015. Cancer Res Treat. 2018; 50:303–316.

4. Korean Breast Cancer Society. Breast Cancer Facts & Figure. Available at.http://www.kbcs.or.kr/sub02/sub04.html. Accessed Oct 14,. 2018.
5. Korean Statistical Information Service. Status of Cancer Screening and Screening by Gender Statistics Korea. Available at.http://kosis.kr. Accessed Aug 14,. 2018.
6. Kolb TM, Lichy J, Newhouse JH. Comparison of the performance of screening mammography, physical examination, and breast US and evaluation of factors that influence them: an analysis of 27,825 patient evaluations.Radiology. 2002; 225:165–175.
7. Berg WA, Blume JD, Cormack JB, Mendelson EB, Lehrer D, Böhm-Vélez M, et al. Combined screening with ultrasound and mammography vs mammography alone in women at elevated risk of breast cancer.JAMA. 2008; 299:2151–2163.
8. Bae MS, Han W, Koo HR, Cho N, Chang JM, Yi A, et al. Characteristics of breast cancers detected by ultrasound screening in women with negative mammograms. Cancer Sci. 2011; 102:1862–1867.

9. Cho N, Moon WK, Chang JM, Yi A, Koo HR, Han BK. Sonographic characteristics of breast cancers detected by supplemental screening US: Comparison with breast cancers seen on screening mammography. Acta Radiol. 2010; 51:969–976.

10. Berg WA. Tailored supplemental screening for breast cancer: what now and what next?AJR Am J Roentgenol. 2009; 192:390–399.
11. Berg WA, Blume JD, Cormack JB, Mendelson EB. Operator dependence of physician-performed whole-breast US: lesion detection and characterization. Radiology. 2006; 241:355–365.

12. Durand MA, Hooley RJ. Implementation of whole-breast screening ultrasonography. Radiol Clin North Am. 2017; 55:527–539.

13. O'Flynn EAM, Fromageau J, Ledger AE, Messa A, D'Aquino A, Schoemaker MJ, et al. Ultrasound tomography evaluation of breast density: a comparison with noncontrast magnetic resonance imaging. Invest Radiol. 2017; 52:343–348.
14. Shin HJ, Kim HH, Cha JH, Park JH, Lee KE, Kim JH. Automated ultrasound of the breast for diagnosis: interobserver agreement on lesion detection and characterization. AJR Am J Roentgenol. 2011; 197:747–754.

15. Grubstein A, Rapson Y, Gadiel I, Cohen M. Analysis of false-negative readings of automated breast ultrasound studies.J Clin Ultrasound. 2017; 45:245–251.
16. Jeh SK, Kim SH, Choi JJ, Jung SS, Choe BJ, Park S, et al. Comparison of automated breast ultrasonography to handheld ultrasonography in detecting and diagnosing breast lesions. Acta Radiol. 2016; 57:162–169.

17. Vourtsis A, Kachulis A. The performance of 3D ABUS versus HHUS in the visualisation and BI-RADS characterisation of breast lesions in a large cohort of 1,886 women.Eur Rad/iiol. 2018; 28:592–601.
18. Li N, Jiang YX, Zhu QL, Zhang J, Dai Q, Liu H, et al. Accuracy of an automated breast volume ultrasound system for assessment of the preoperative extent of pure ductal carcinoma in situ: comparison with a conventional handheld ultrasound examination.Ultrasound Med Biol. 2013; 39:2255–2263.
19. Isobe S, Tozaki M, Yamaguchi M, Ogawa Y, Homma K, Satomi R, et al. Detectability of breast lesions under the nipple using an automated breast volume scanner: comparison with handheld ultrasonography. Jpn J Rad iol. 2011; 29:361–365.

20. Kelly KM, Dean J, Comulada WS, Lee SJ. Breast cancer detection using automated whole breast ultrasound and mammography in radiographically dense breasts. Eur Radiol. 2010; 20:734–742.

21. Giuliano V, Giuliano C. Improved breast cancer detection in asymptomatic women using 3D-automated breast ultrasound in mammographically dense breasts.Clin Imaging. 2013; 37:480–486.
22. Brem RF, Tabár L, Duffy SW, Inciardi MF, Guingrich JA, Hashimoto BE, et al. Assessing improvement in detection of breast cancer with three-dimensional automated breast US in women with dense breast tissue: the SomoInsight Study. Radiology. 2015; 274:663–673.

23. Wilczek B, Wilczek HE, Rasouliyan L, Leifland K. Adding 3D automated breast ultrasound to mammography screening in women with heterogeneously and extremely dense breasts: Report from a hospital-based, high-volume, single-center breast cancer screening program. Eur J Radiol. 2016; 85:1554–1563.

24. Arleo EK, Saleh M, Ionescu D, Drotman M, Min RJ, Hentel K. Recall rate of screening ultrasound with automated breast volumetric scanning (ABVS) in women with dense breasts: a first quarter experience.Clin Imaging. 2014; 38:439–444.
25. Giger ML, Inciardi MF, Edwards A, Papaioannou J, Drukker K, Jiang Y, et al. Automated breast ultrasound in breast cancer screening of women with dense breasts: reader study of mammography-negative and mammography-positive cancers.AJR Am J Roentgenol. 2016; 206:1341–1350.
26. Chang JM, Cha JH, Park JS, Kim SJ, Moon WK. Automated breast ultrasound system (ABUS): reproducibility of mass localization, size measurement, and characterization on serial examinations.Acta Radiol. 2015; 56:1163–1170.
27. Chang JM, Moon WK, Cho N, Park JS, Kim SJ. Breast cancers initially detected by handheld ultrasound: detection performance of radiologists using automated breast ultrasound data.Acta Radiol. 2011; 52:8–14.
28. Choi EJ, Choi H, Park EH, Song JS, Youk JH. Evaluation of an automated breast volume scanner according to the fifth edition of BI-RADS for breast ultrasound compared with handheld ultrasound.Eur J Radiol. 2018; 99:138–145.
29. Barr RG, DeVita R, Destounis S, Manzoni F, De Silvestri A, Tinelli C. Agreement between an automated volume breast scanner and handheld ultrasound for diagnostic breast examinations.J Ultrasound Med. 2017; 36:2087–2092.
30. Lin X, Wang J, Han F, Fu J, Li A. Analysis of eighty-one cases with breast lesions using automated breast volume scanner and comparison with handheld ultrasound. Eur J Radiol. 2012; 81:873–878.

31. Schmachtenberg C, Fischer T, Hamm B, Bick U. Diagnostic performance of automated breast volume scanning (ABVS) compared to handheld ultrasonography with breast MRI as the gold standard. Acad Radiol. 2017; 24:954–961.

32. Wang HY, Jiang YX, Zhu QL, Zhang J, Dai Q, Liu H, et al. Differentiation of benign and malignant breast lesions: a comparison between automatically generated breast volume scans and handheld ultrasound examinations. Eur J Radiol. 2012; 81:3190–3200.

33. Wang X, Huo L, He Y, Fan Z, Wang T, Xie Y, et al. Early prediction of pathological outcomes to neoadjuvant chemotherapy in breast cancer patients using automated breast ultrasound.Chin J Cancer Res. 2016; 28:478–485.
Go to : 
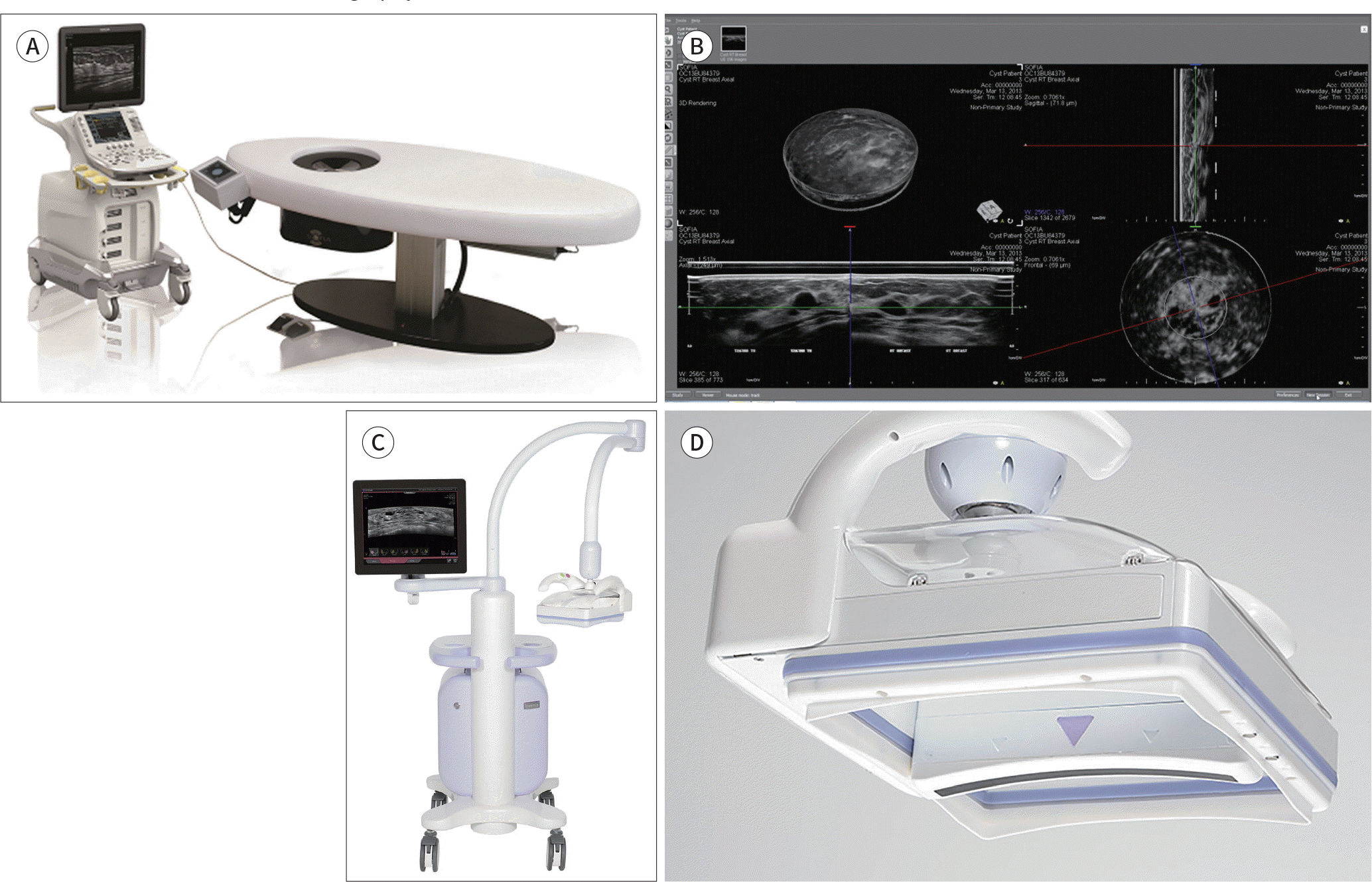 | Fig. 1.An overview of the ABUS system. The ABUS system with a prone method scanner (A) can fully scan each breast in 30 seconds. A 92-mm linear ultrasound probe automatically rotates around the nipple in a cone-shaped scan as the patient lies on the scanning bed. The review workstation displays multiplane images, including 2D axial and sagittal views, as well as 3D images (B) (images obtained from the Hitachi website). The ABUS system with a supine method scanner (C) scans the entire breast volume on medial, anteroposterior, and lateral views. Each examination, including both breasts, takes approximately 15 minutes. The gentle shape of the Reverse Curve™ (InveniaTM ABUS, Automated Breast Ultrasound System; GE Healthcare, Sunnyvale, CA, USA) transducer (D) follows the natural contour of the breast, providing patient comfort, full contact, and comprehensivecoverage. The 15-cm field-of-view transducer is easy to position and maintains even compression while scanning. ABUS = automated breast ultrasonography |
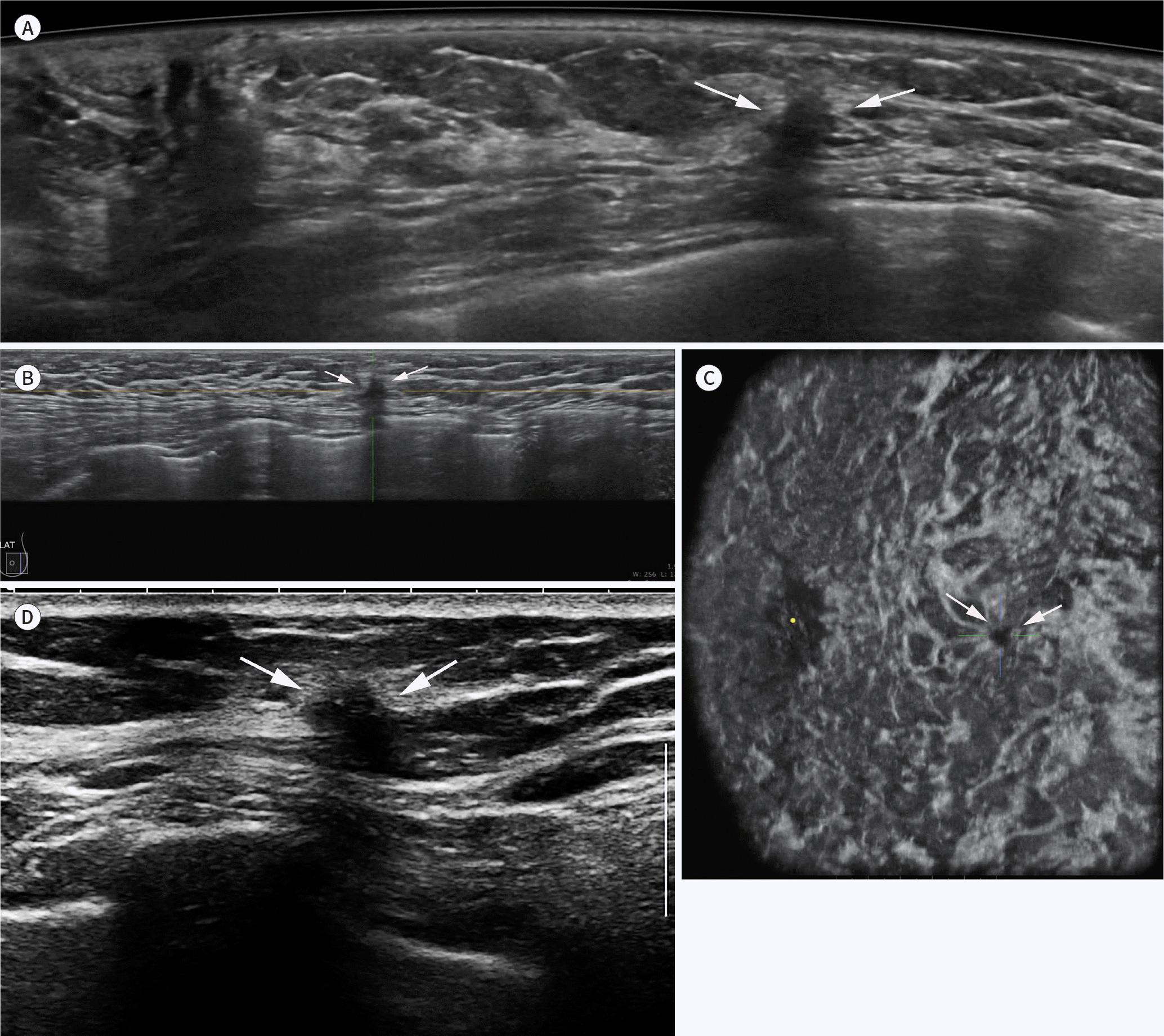 | Fig. 2.Screening results from a 55-year-old woman. ABUS is used to screen mammographically dense breast tissue. Axial (A), sagittal (B), and coronal (C) ABUS views show a spiculated, irregular, hypoechoic mass (arrows) with shadowing at the three o'clock position in the left breast. A handheld ultrasound examination of the biopsied specimen (D) shows similar features of the mass (arrows), which was confirmed as invasive ductal cancer [pT1cN0(sn)]. ABUS = automated breast ultrasonography |
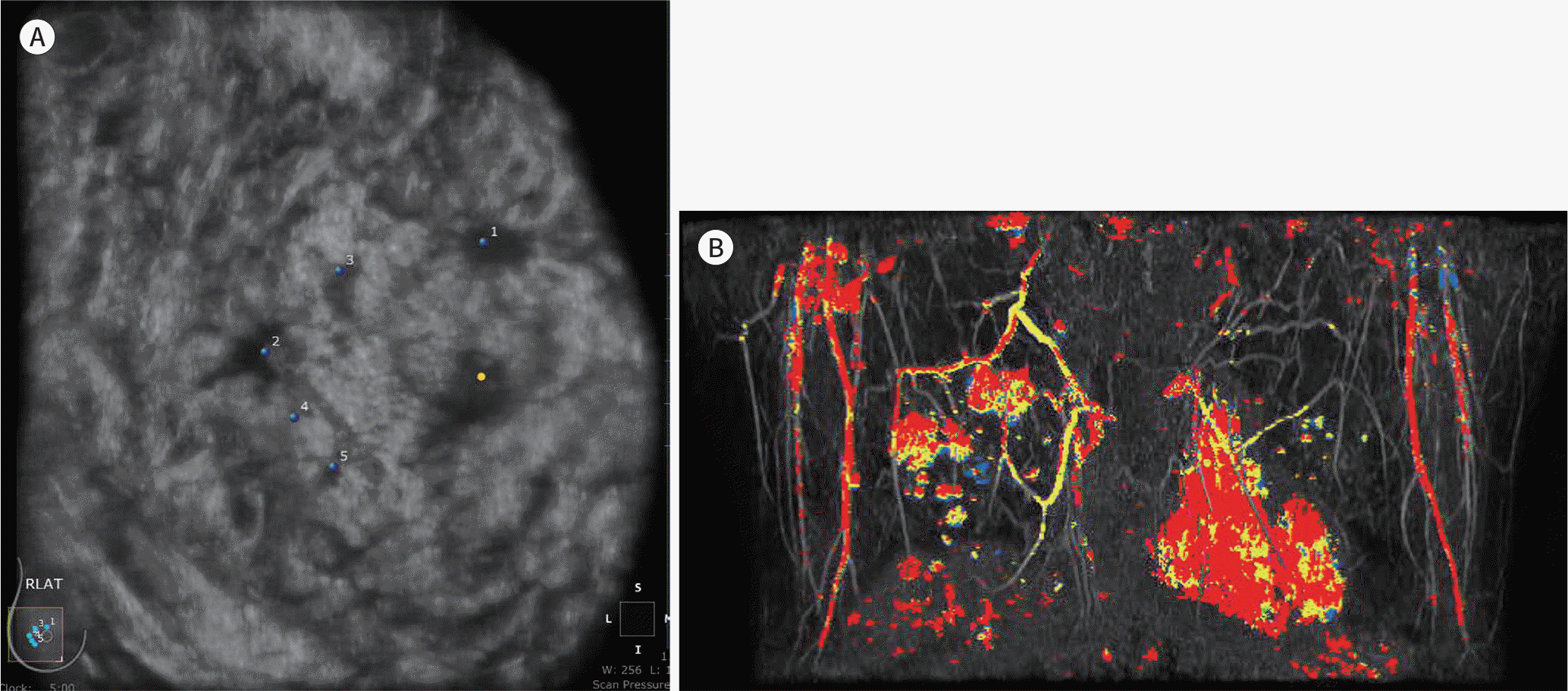 | Fig. 3.A 53-year-old woman with invasive ductal cancer. Diagnostic ABUS was performed for a recently confirmed invasive ductal cancer. The coronal ABUS image (A) shows multifocal and multicentric cancers in the right breast, identical to those on the maximum intensity projection image of post-gadolinium-enhanced breast MRI (B). ABUS = automated breast ultrasonography |
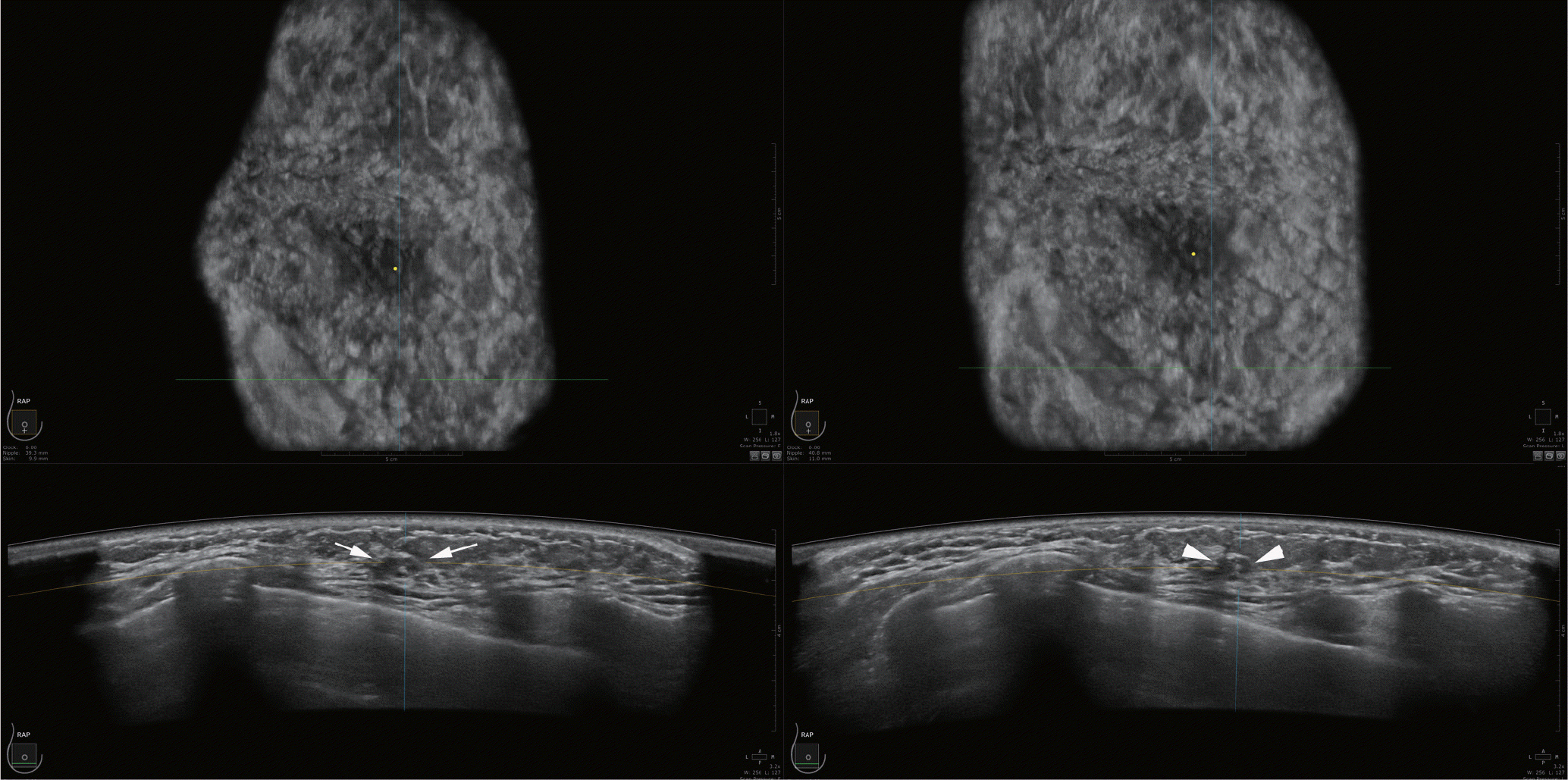 | Fig. 4.Follow-up of a 42-year-old woman with multiple probably benign masses. A side-by-side comparison with an ultrasound image taken six months previously. The mass (arrows) shows no significant change compared to the previous image (arrowheads). |
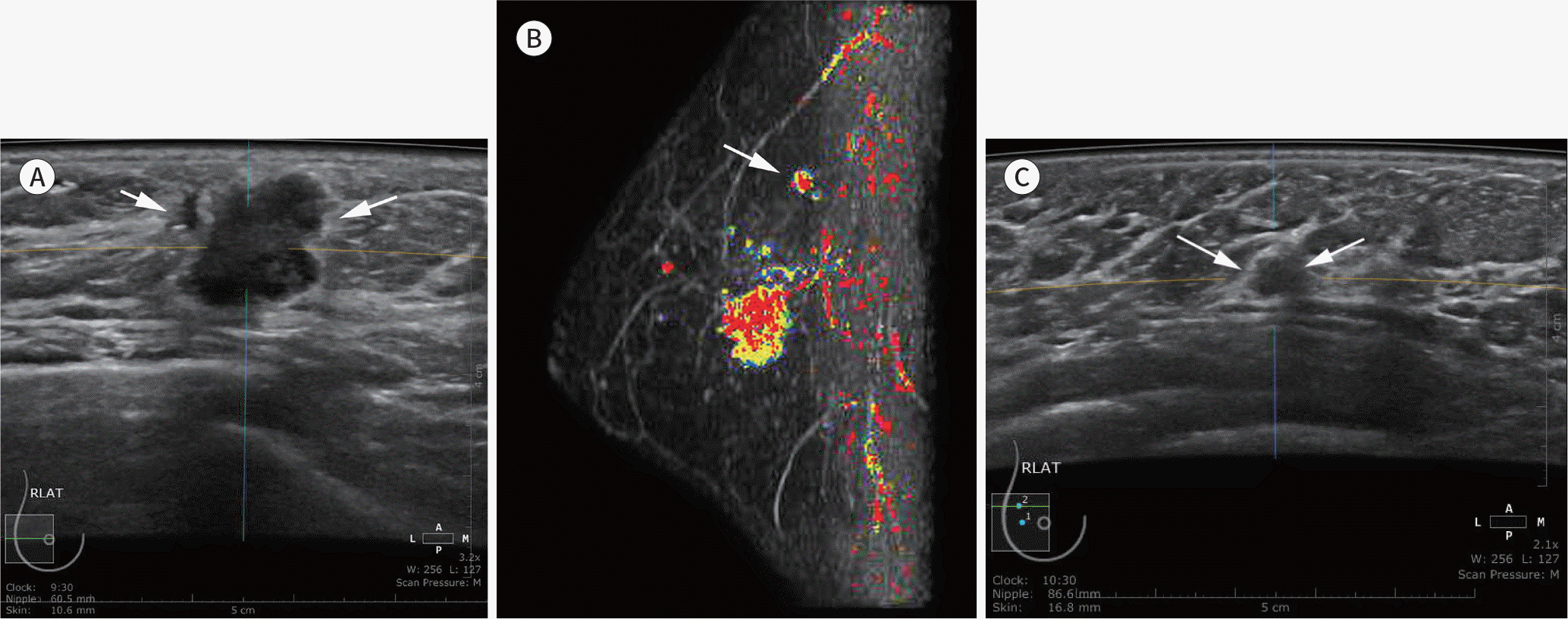 | Fig. 5.A 43-year-old woman with invasive ductal cancer. Diagnostic ABUS was performed for a recently confirmed invasive ductal cancer. An axial ABUS image (A) shows a microlobulated, irregular, hypoechoic mass (arrows) at the 9 o'clock position in the right breast, (B) a preoperative MR examination identified another mass (arrow) in the upper quadrant of the right breast on a computer-aided diagnosis maximum intensity projection image of post-gadolinium-enhanced breast MRI (B). A reinterpreted ABUS image (C) shows a 0.5-cm indistinct, oval, hypoechoic mass (arrows), confirmed as another focus of invasive ductal cancer. ABUS = automated breast ultrasonography |
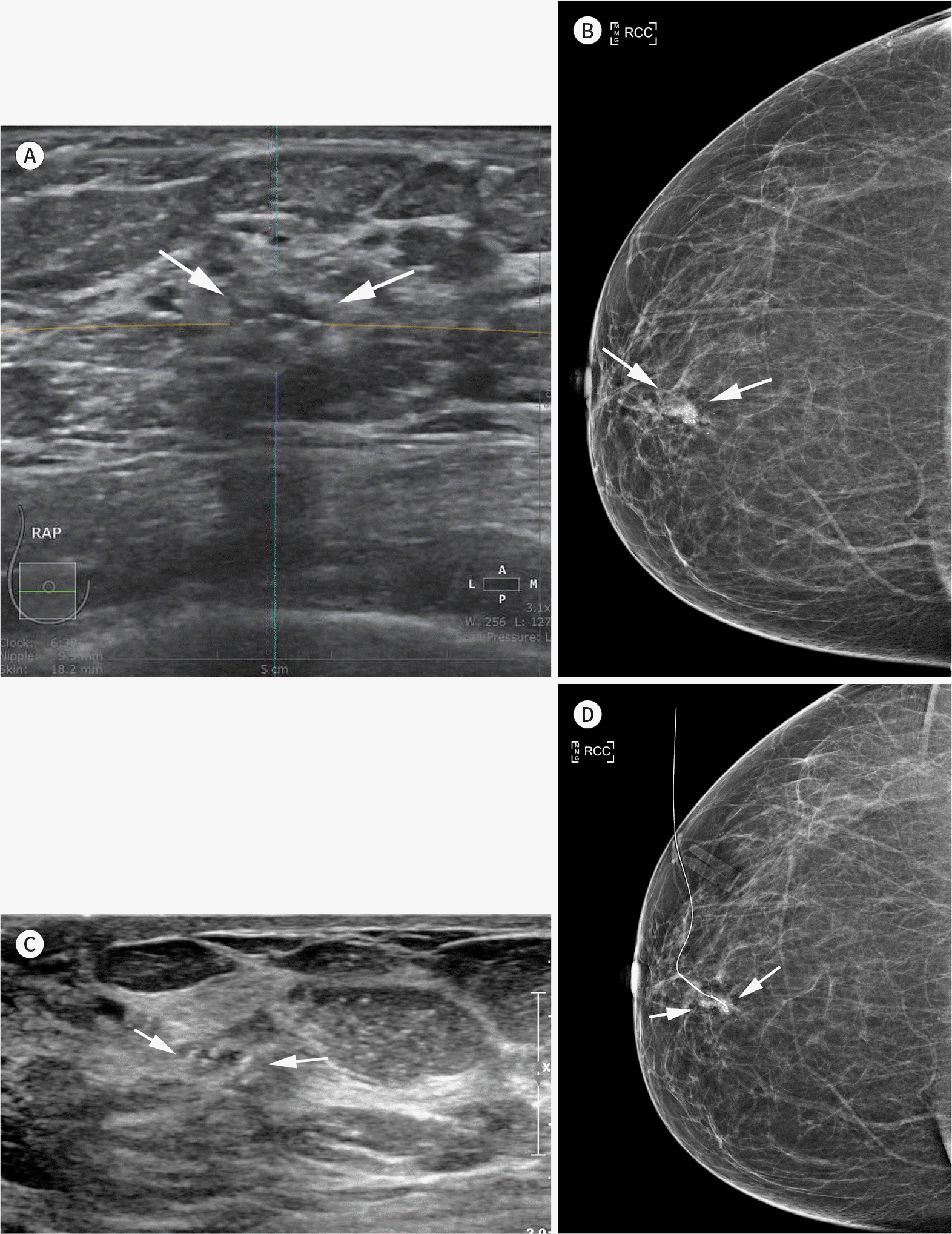 | Fig. 6.A 67-year-old woman with DCIS. Diagnostic ABUS was performed for a recently confirmed DCIS. An axial ABUS image (A) shows an indistinct, oval, heterogeneous echoic mass (arrows) in the subareolar area of the right breast. Right craniocaudal mammography (B) shows an indistinct, irregular, high-density mass with internal microcalcifications (arrows). Handheld ultrasound-assisted localization (C) shows microcalcifications in an isoechoic mass (arrows), which is difficult to recognize without correlation with mammography. Post-localization mammography (D) confirmed ultrasonographic calcifications correlated with a mammographic lesion (arrows). ABUS = automated breast ultrasonography, DCIS = ductal carcinoma in situ |
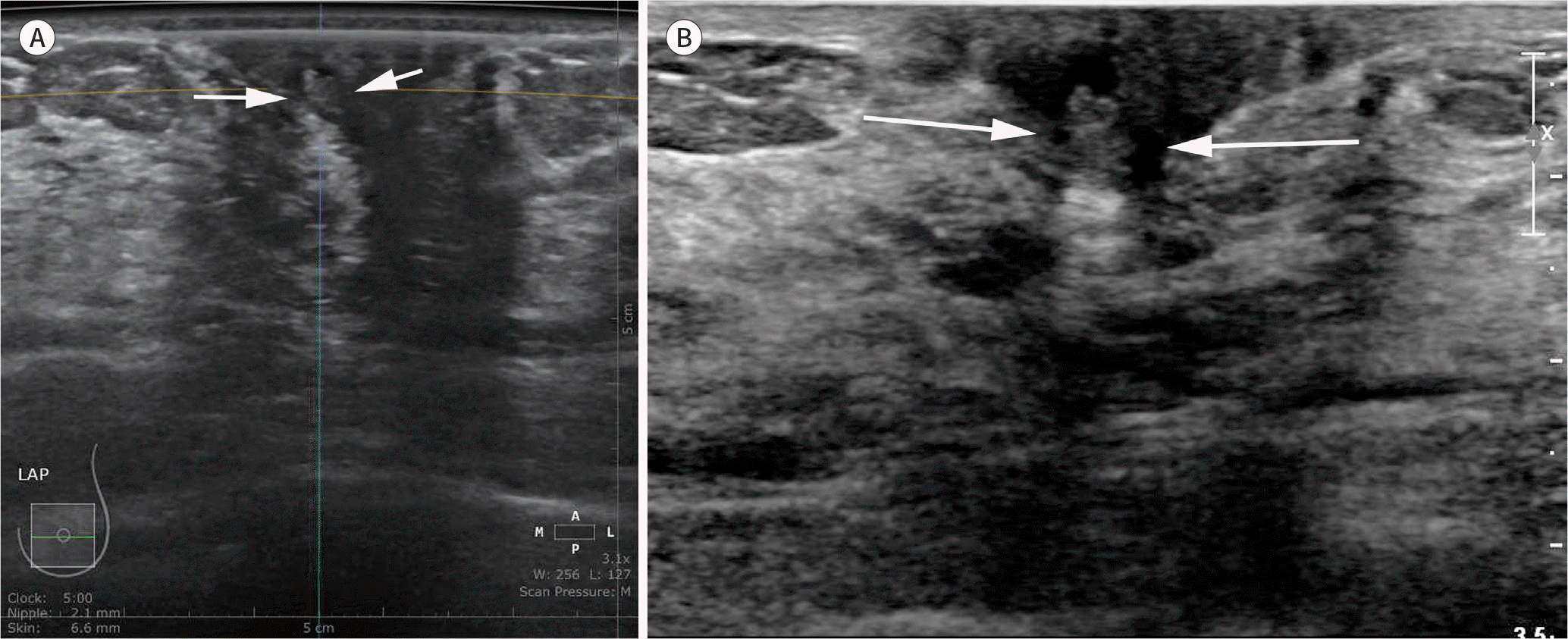 | Fig. 7.A 63-year-old woman with left nipple discharge. Diagnostic ABUS was performed for left nipple discharge. An axial ABUS image (A) shows an indistinct, oval, isoechoic solid and cystic mass (arrows) in the subareolar area of the left breast. The mass (arrows) is identical to that obtained using handheld ultrasound examination (B); however, the subareolar lesion is not commonly missed by ABUS due to nipple shadowing and therefore requires careful evaluation. ABUS = automated breast ultrasonography |
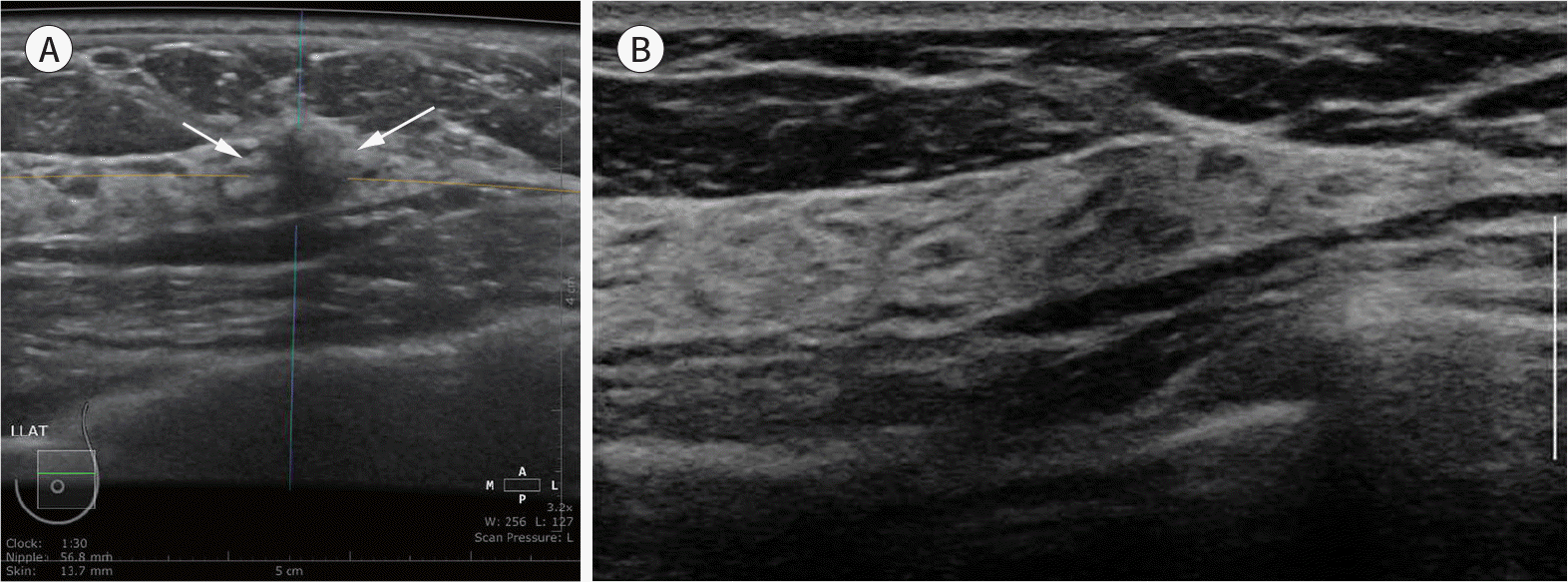 | Fig. 8.Screening of a 59-year-old woman. ABUS was performed to screen dense breasts. An axial ABUS image (A) shows an indistinct, irregular, hypoechoic mass (arrows) in the upper outer quadrant of the left breast, which was not detected by a handheld ultrasound examination (B). This case was recalled due to a posterior shadowing artifact. ABUS = automated breast ultrasonography |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download