1. Wikipedia. Informatics. Available at.https://en.wikipedia.org/wiki/Informatics. Accessed Jan 22,. 2019.
2. Korean Society of imaging Informatics in Medicine. Greeting. Available at.https://ksiim.org/site/introduce/greeting/new. Accessed Jan 22,. 2019.
3. Park EJ, Kim SI. The guideline for the standardization of the PACS Components.J Kor PACS Soc. 1998; 4:91–99.
4. Park HJ, Kim JH, Kim SI. An introduction of DICOM 3.0.J Kor PACS Soc. 1998; 4:145–166.
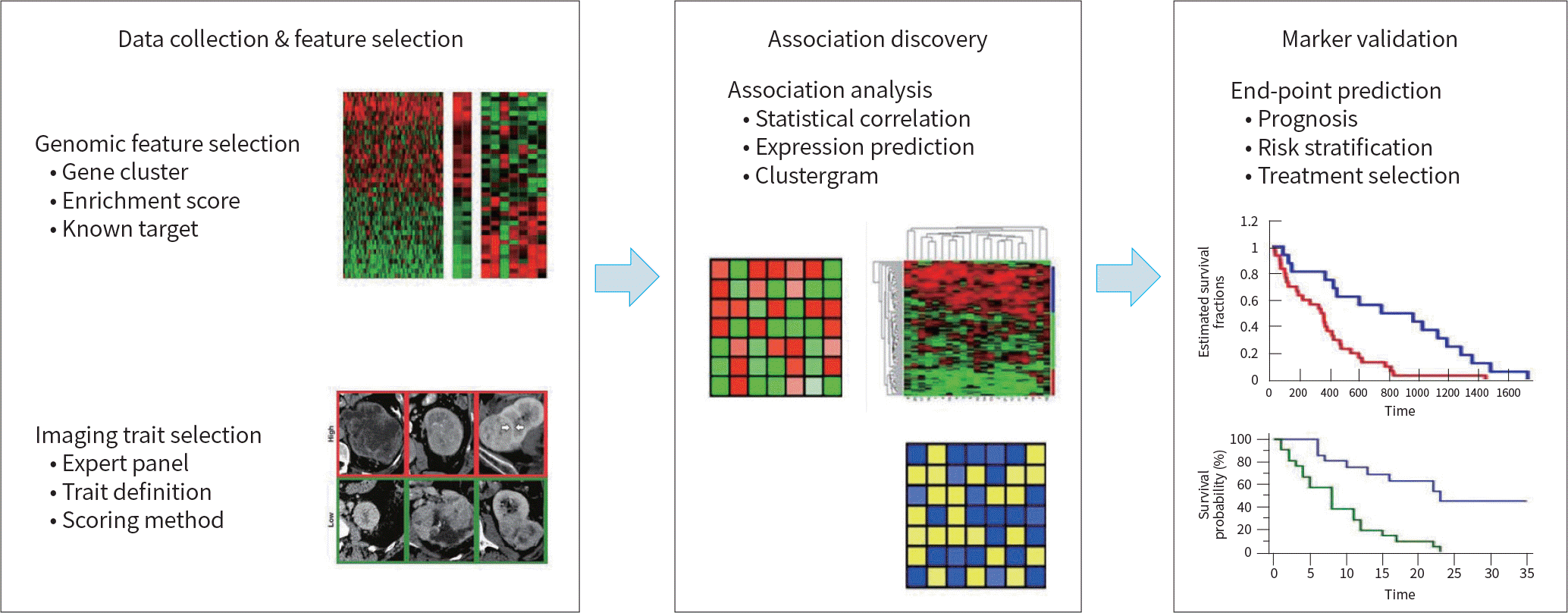
5. Mazurowski MA. Radiogenomics: what it is and why it is important.J Am Coll Radiol. 2015; 12:862–866.
6. Segal E, Sirlin CB, Ooi C, Adler AS, Gollub J, Chen X, et al. Decoding global gene expression programs in liver cancer by noninvasive imaging.Nat Biotechnol. 2007; 25:675–680.
7. Woo BY, Lee ME, Kim JH. Repeatability of gene set enrichment analysis in radiogenomics.J Kor Soc Imag Inf or Med. 2016; 22:29–37.
8. Lee ME, Kim JH. Opportunities and challenges in radiogenomics: imaging phenotype analysis for brain tumor. J Kor Soc Imag Infor Med. 2014; 20:19–26.
9. Diehn M, Nardini C, Wang DS, McGovern S, Jayaraman M, Liang Y, et al. Identification of noninvasive imaging surrogates for brain tumor gene-expression modules. Proc Natl Acad Sci U S A. 2008; 105:5213–5218.

10. Zinn PO, Mahajan B, Sathyan P, Singh SK, Majumder S, Jolesz FA, et al. Radiogenomic mapping of edema/cellular invasion MRI-phenotypes in glioblastoma multiforme.PLoS One. 2011; 6:e25451.
11. Yamamoto S, Maki DD, Korn RL, Kuo MD. Radiogenomic analysis of breast cancer using MRI: a preliminary study to define the landscape. AJR Am J Roentgenol. 2012; 199:654–663.

12. Gevaert O, Xu J, Hoang CD, Leung AN, Xu Y, Quon A, et al. Non-small cell lung cancer: identifying prognostic imaging biomarkers by leveraging public gene expression microarray data–methods and preliminary results. Radiology. 2012; 264:387–396.

13. Karlo CA, Di Paolo PL, Chaim J, Hakimi AA, Ostrovnaya I, Russo P, et al. Radiogenomics of clear cell renal cell carcinoma: associations between CT imaging features and mutations.Radiology. 2014; 270:464–471.
14. Jamshidi N, Diehn M, Bredel M, Kuo MD. Illuminating radiogenomic characteristics of glioblastoma multi-forme through integration of MR imaging, messenger RNA expression, and DNA copy number variation.Radiology. 2014; 270:1–2.
15. Yamamoto S, Korn RL, Oklu R, Migdal C, Gotway MB, Weiss GJ, et al. ALK molecular phenotype in non-small cell lung cancer: CT radiogenomic characterization.Rad/iiology. 2014; 272:568–576.
16. Yamamoto S, Han W, Kim Y, Du L, Jamshidi N, Huang D, et al. Breast cancer: radiogenomic biomarker reveals associations among dynamic contrast-enhanced MR imaging, long noncoding RNA, and metastasis. Radiology. 2015; 275:384–392.

17. Cancer Imaging Archive. TCIA collections. Available at.http://www.cancerimagingarchive.net/. Accessed Jan 22,. 2019.
18. Gillies RJ, Kinahan PE, Hricak H. Radiomics: images are more than pictures, they are data.Radiology. 2016; 278:563–577.
19. Lee M, Woo B, Kuo MD, Jamshidi N, Kim JH. Quality of radiomic features in glioblastoma multiforme: impact of semiautomated tumor segmentation software. Korean J Radiol. 2017; 18:498–509.

20. Balagurunathan Y, Gu Y, Wang H, Kumar V, Grove O, Hawkins S, et al. Reproducibility and prognosis of quantitative features extracted from CT images. Transl Oncol. 2014; 7:72–87.

21. Kim H, Park CM, Lee SM, Lee HJ, Goo JM. A comparison of two commercial volumetry software programs in the analysis of pulmonary groundglass nodules: segmentation capability and measurement accuracy. Kor ean J Radiol. 2013; 14:683–691.

22. Ryoo I, Choi SH, Kim JH, Sohn CH, Kim SC, Shin HS, et al. Cerebral blood volume calculated by dynamic susceptibility contrast-enhanced perfusion MR imaging: preliminary correlation study with glioblastoma genetic profiles.PLoS One. 2013; 8:e71704.
23. Egger J, Kapur T, Fedorov A, Pieper S, Miller JV, Veeraraghavan H, et el. GBM volumetry using the 3D Slicer medical image computing platform. Sci Rep. 2013; 3:1364.
24. Zhu Y, Young GS, Xue Z, Huang RY, You H, Setayesh K, et al. Semi-automatic segmentation software for quantitative clinical brain glioblastoma evaluation.Acad Radiol. 2012; 19:977–985.
25. Kim JW, Kim JH. Review of evaluation metrics for 3D medical image segmentation.J Kor Soc Imag Infor Med. 2017; 23:14–20.
26. Lao J, Chen Y, Li ZC, Li Q, Zhang J, Liu J, et al. A deep learning-based radiomics model for prediction of survival in glioblastoma multiforme. Sci Rep. 2017; 7:10353.

27. Limkin EJ, Sun R, Dercle L, Zacharaki EI, Robert C, Reuzé S, et al. Promises and challenges for the implementation of computational medical imaging (radiomics) in oncology.Ann Oncol. 2017; 28:1191–1206.
28. Lambin P, Rios-Velazquez E, Leijenaar R, Carvalho S, Van Stiphout RG, Granton P, et al. Radiomics: extracting more information from medical images using advanced feature analysis.Eur J Cancer. 2012; 48:441–446.
29. Aerts HJ, Velazquez ER, Leijenaar RT, Parmar C, Grossmann P, Carvalho S, et al. Decoding tumour phenotype by noninvasive imaging using a quantitative radiomics approach.Nat Commun. 2014; 5:4006.
30. Coroller TP, Grossmann P, Hou Y, Rios Velazquez E, Leijenaar RT, Hermann G, et al. CT-based radiomic signature predicts distant metastasis in lung adenocarcinoma. Radiother Oncol. 2015; 114:345–350.

31. Hawkins S, Wang H, Liu Y, Garcia A, Stringfield O, Krewer H, et al. Predicting malignant nodules from screening CT scans.J Thorac Oncol. 2016; 11:2120–2128.
32. Wu W, Parmar C, Grossmann P, Quackenbush J, Lambin P, Bussink J, et al. Exploratory study to identify radiomics classifiers for lung cancer histology.Front Oncol. 2016; 6:71.
33. Kickingereder P, Burth S, Wick A, Götz M, Eidel O, Schlemmer HP, et al. Radiomic profiling of glioblastoma: identifying an imaging predictor of patient survival with improved performance over established clinical and radiologic risk models. Radiology. 2016; 280:880–889.

34. Huang YQ, Liang CH, He L, Tian J, Liang CS, Chen X, et al. Development and validation of a radiomics nomogram for preoperative prediction of lymph node metastasis in colorectal cancer.J Clin Oncol. 2016; 34:2157–2164.
35. Aerts HJ, Grossmann P, Tan Y, Oxnard GR, Rizvi N, Schwartz LH, et al. Defining a radiomic response phenotype: a pilot study using targeted therapy in NSCLC. Sci Rep. 2016; 6:33860.

36. Michoux N, Van den Broeck S, Lacoste L, Fellah L, Galant C, Berlière M, et al. Texture analysis on MR images helps predicting non-response to NAC in breast cancer.BMC Cancer. 2015; 15:574.
37. Nie K, Shi L, Chen Q, Hu X, Jabbour SK, Yue N, et al. Rectal cancer: assessment of neoadjuvant chemoradiation outcome based on radiomics of multiparametric MRI.Clin Cancer Res. 2016; 22:5256–5264.
38. Fehr D, Veeraraghavan H, Wibmer A, Gondo T, Matsumoto K, Vargas HA, et al. Automatic classification of prostate cancer Gleason scores from multiparametric magnetic resonance images. Proc Natl Acad Sci U S A. 2015; 112:E6265–E6273.

39. Li H, Zhu Y, Burnside ES, Drukker K, Hoadley KA, Fan C, et al. MR imaging radiomics signatures for predicting the risk of breast cancer recurrence as given by research versions of MammaPrint, Oncotype DX, and PAM50 gene assays. Radiology. 2016; 281:382–391.

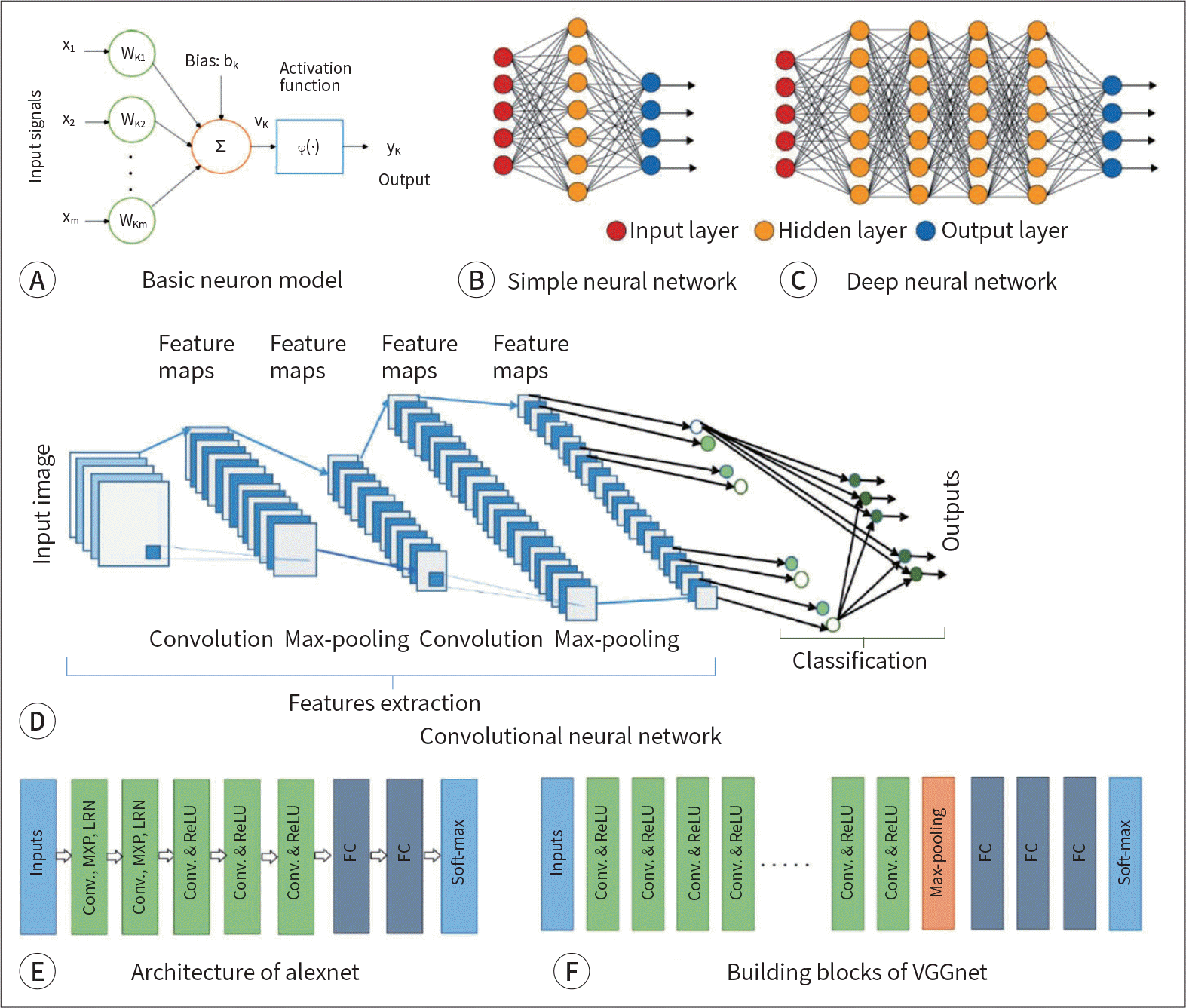
40. Lee JG, Jun S, Cho YW, Lee H, Kim GB, Seo JB, et al. Deep learning in medical imaging: general overview. Korean J Radiol. 2017; 18:570–584.

41. Alom MZ, Taha TM, Yakopcic C, Westberg S, Sidike P, Nasrin MS, et al. The history began from alexnet: a comprehensive survey on deep learning approaches.arXiv preprint. 2018; arXiv:1803. .01164.
42. Sahiner B, Pezeshk A, Hadjiiski LM, Wang X, Drukker K, Cha KH, et al. Deep learning in medical imaging and radiation therapy.Med Phys. 2019; 46:e1–e36.
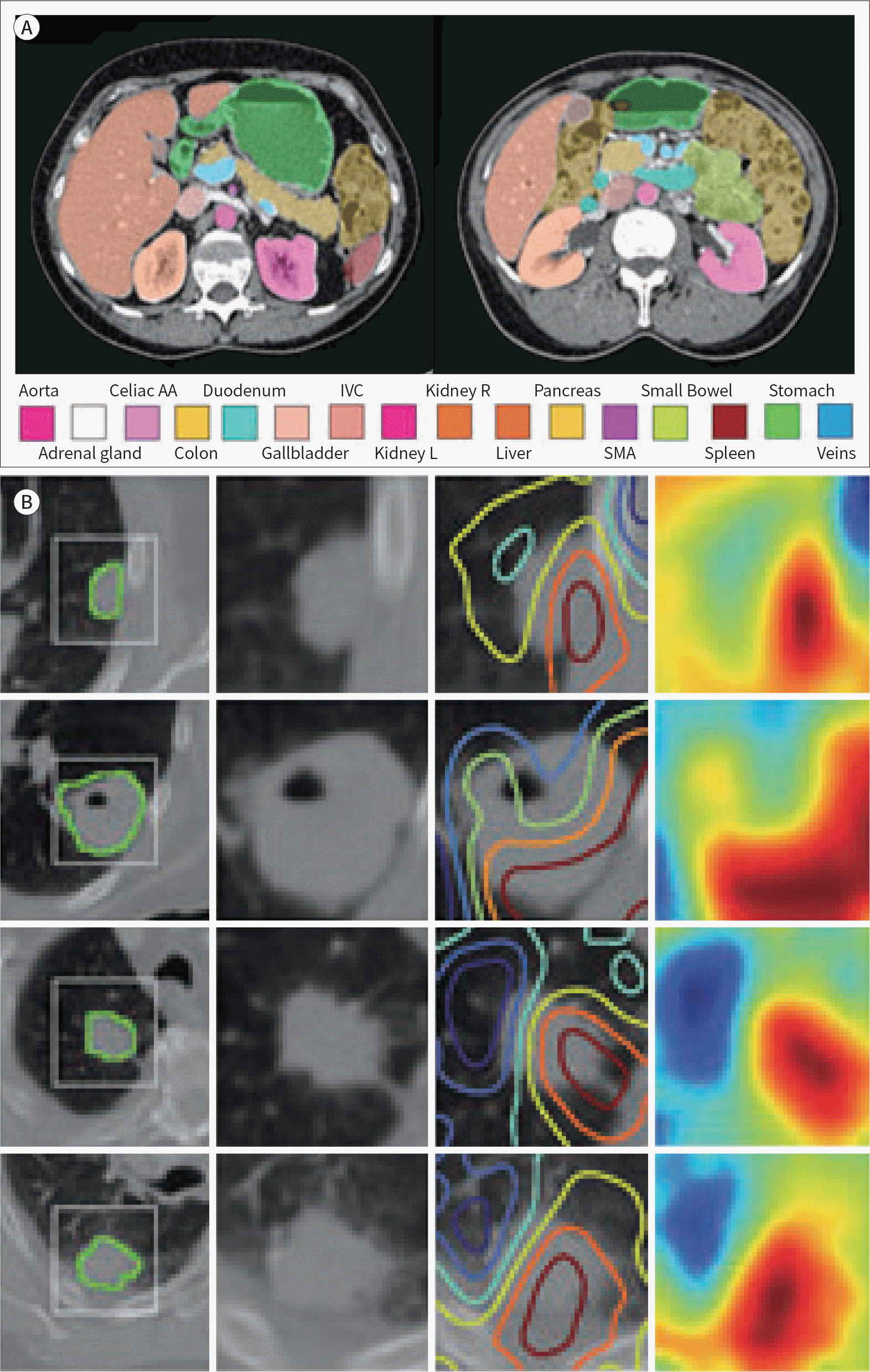
43. Hu P, Wu F, Peng J, Bao Y, Chen F, Kong D. Automatic abdominal multiorgan segmentation using deep convolutional neural network and time-implicit level sets.Int J Comput Assist Radiol Surg. 2017; 12:399–411.
44. Dolz J, Desrosiers C, Ben Ayed I. 3D fully convolutional networks for subcortical segmentation in MRI: A large-scale study. NeuroImage. 2018; 170:456–470.

45. Cheng R, Roth HR, Lay N, Lu L, Turkbey B, Gandler W, et al. Automatic magnetic resonance prostate segmentation by deep learning with holistically nested networks.J Med Imaging (Bellingham). 2017; 4:041302.
46. Wang S, Zhou M, Liu Z, Liu Z, Gu D, Zang Y, et al. Central focused convolutional neural networks: developing a data-driven model for lung nodule segmentation.Med Image Anal. 2017; 40:172–183.
47. Alex V, Vaidhya K, Thirunavukkarasu S, Kesavadas C, Krishnamurthi G. Semisupervised learning using denoising autoencoders for brain lesion detection and segmentation.J Med Imag/iing (Bellingham). 2017; 4:041311.
48. Zhang R, Huang L, Xia W, Zhang B, Qiu B, Gao X. Multiple supervised residual network for osteosarcoma segmentation in CT images. Comput Med Imaging Graph. 2018; 63:1–8. ˇ.
49. Payer C, S tern D, Bischof H, Urschler M. Regressing heatmaps for multiple landmark localization using CNNs. In International Conference on Medical Image Computing and Computer-Assisted Intervention. Cham:. Springer;2006. p. 230–238.
50. Zhang J, Liu M, Shen D. Detecting anatomical landmarks from limited medical imaging data using two-stage task-oriented deep neural networks.IEEE Trans Image Process. 2017; 26:4753–4764.
51. Harrison AP, Xu Z, George K, Lu L, Summers RM, Mollura DJ.Progressive and multi-path holistically nested neural networks for pathological lung segmentation from CT images. In International Conference on Medi-cal Image Computing and Computer-Assisted Intervention. Cham:. Springer;2006. p. 621–629.
52. Rajpurkar P, Irvin J, Ball RL, Zhu K, Yang B, Mehta H, et al. Deep learning for chest radiograph diagnosis: a retrospective comparison of the CheXNeXt algorithm to practicing radiologists.PLoS Med. 2018; 15:e1002686.
53. Nam JG, Park S, Hwang EJ, Lee JH, Jin KN, Lim KY, et al. Development and validation of deep learning-based automatic detection algorithm for malignant pulmonary nodules on chest radiographs.Radiology. 2019; 290:218–228.
54. Wang Y, Zhou Y, Tang P, Shen W, Fishman EK, Yuille AL. Training multiorgan segmentation networks with sample selection by relaxed upper confident bound. arXiv preprint. 2018; arXiv:1804. .02595.

55. Hosny A, Parmar C, Coroller TP, Grossmann P, Zeleznik R, Kumar A, et al. Deep learning for lung cancer prognostication: a retrospective multicohort radiomics study.PLoS Med. 2018; 15:e1002711.
56. Wang H, Xu Z, Fujita H, Liu S. Towards felicitous decision making: an overview on challenges and trends of Big Data. Inf Sci. 2016; 367–368:747–765.

57. Jain A. Healthcare data analytics: the 5 Vs of big data. Available at.https://www.ibm.com/blogs/watson-health/the-5-vs-of-big-data/. Accessed Feb 10,. 2019.
58. Neugebauer R. Trends in industrie 4.0. Avaiable at.http://www.fraunhofer.jp/content/dam/japan/ja/docu-ments/media/publication/Trends-in-Industrie-40.pdf. Accessed Feb 10,. 2019.
59. Donoho D. High-dimensional data analysis: the curses and blessings of dimensionality in Mathematical Challenges of the 21st Century. 2000. Available at:. http://statweb.stanford.edu/∼donoho/Lectures/AMS2000/Curses.pdf. Accessed Feb 10,. 2019.
60. Daniel N. 5 fixes for your failing big data initiatives. Available at.https://www.forbes.com/sites/danielnewman/2018/06/28/5-fixes-for-your-failing-big-data-initiatives/#57c08a845a0b. Accessed Feb 10,. 2019.
61. Morris MA, Saboury B, Burkett B, Gao J, Siegel EL. Reinventing radiology: big data and the future of medical imaging.J Thorac Imaging. 2018; 33:4–16.
62. ICD10Data. ICD-10. Available at.https://www.icd10data.com/ICD10CM/Codes. Accessed Feb 10,. 2019.
63. Wikipedia. SNOMED CT.kr. Available at.https://en.wikipedia.org/wiki/SNOMED_CT. Accessed Feb 10,. 2019.
64. RSNA Informatics. RadLex. Available at.http://www.radlex.org/. Accessed Feb 10,. 2019.
65. Jamshidi N, Jonasch E, Zapala M, Korn RL, Aganovic L, Zhao H, et al. The radiogenomic risk score: construction of a prognostic quantitative, noninvasive image-based molecular assay for renal cell carcinoma.Radiol-ogy. 2015; 277:114–123.
66. Traverso A, Wee L, Dekker A, Gillies R. Repeatability and reproducibility of radiomic features: a systematic review. Int J Radiat Oncol Biol Phys. 2018; 102:1143–1158.

67. Wikipedia. Explainable artificial intelligence. Available at.https://en.wikipedia.org/wiki/Explainable_Artifi-cial_Intelligence. Accessed Jan 22,. 2019.
68. Abajian AC, Levy M, Rubin DL. Informatics in radiology: improving clinical work flow through an AIM database: a sample web-based lesion tracking application. Radiographics. 2012; 32:1543–1552.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download