Abstract
Purpose
Hyperthermic intraperitoneal chemotherapy (HIPEC) is a novel treatment option for peritoneal surface malignancies. Due to cytotoxic effects of chemotherapeutic agents, anastomosis healing can be impaired and lead to leakage rates higher than conventional intestinal surgery. In this experimental study, we aimed to investigate the effects of platelet-rich plasma (PRP) on colonic anastomosis in rats that received HIPEC with oxaliplatin.
Methods
Thirty rats were divided into 3 groups. Group 1 was determined as control group and hyperthermic saline perfusion was performed after colon anastomosis. In group 2, colon anastomosis then hyperthermic oxaliplatin perfusion was performed. In the last group, the colonic anastomosis was enhanced by PRP gel and then hyperthermic oxaliplatin perfusion was performed. All the rats were reoperated on postoperative day 7 and anastomotic bursting pressure values were recorded. Tissue samples were taken for hydroxyproline assay and histopathological examination.
Results
Control group had higher anastomotic bursting pressure value than group 2 and group 3 (P < 0.001). There were significant differences in anastomotic bursting pressure between groups 2 and 3 (P < 0.001). Group 2 had significantly lower hydroxyproline levels than group 3 and control group (P < 0.001). Histopathological examination revealed that PRP application reduced inflammatory response.
Despite developing diagnostic tools and screening programs, approximately 4%–7% of patients with colon cancer have peritoneal carcinomatosis (PC) at the time of diagnosis [1]. Metachronous peritoneal metastasis can also be seen in 4% to 50% of patients during follow-up [234]. In the past, PC was accepted as the terminal stage. However, recently a new treatment modality called ‘hyperthermic intraperitoneal chemotherapy (HIPEC) combined with cytoreductive surgery (CRS)’ has provided prolonged survival rates with acceptable mortality and morbidity rates [56]. Anastomotic leakage rates have been found to be higher in CRS plus HIPEC compared to conventional colorectal surgery ranging between 10%–25% in various studies, which is why stomal diversion is required in such patients [7891011].
Platelet-rich plasma (PRP) contains numerous plateletderived growth factors which improve wound healing and have been widely used in various surgical procedures. Promising experimental studies have been reported regarding PRP intestinal anastomotic healing and PRP's application [121314].
In this experimental animal study, we aimed to investigate whether PRP application has favorable effects on colonic anastomosis in rats when performed with oxaliplatin-based HIPEC.
This study was conducted at the Experimental Animal Application and Research Center of Ondokuz Mayıs University upon the approval of the Local Ethical Committee of Experimental Animal of Ondokuz Mayıs University (approval number: 2017/51). Thirty-five male Wistar-Albino rats were used. Rats were maintained at 22℃ ± 2℃ with a 12-hour light/dark cycle at 40%–50% humidity. Rats were allowed free access to water and standard laboratory rat pellets.
Five rats were selected as donors and used for PRP preparation. Blood samples were obtained by cardiac puncture and 8.5 mL of blood from each donor was drawn into the acid-citrate-dextrose tubes (BD Vacutainer, BD, Franklin Lakes, NJ, USA). The tubes were then centrifuged at 2,500 rpm and 22℃ for 10 minutes. After the first spin, 3 layers were seen as follows; (1) light yellow plasma layer, (2) buffy coat layer, and (3) red blood cells layer (Fig. 1A). Plasma and buffy coat layers were taken carefully and drawn into another tube, then centrifuged at 3,500 rpm and 22℃ for 15 minutes. Finally, 1-mL PRP was harvested and mixed with 1-mL thrombine (Human Thrombin Lyophilized, Baxter, Cherry Hill, NJ, USA) and 0.5-mL calcium chloride in order to obtain the gel form of PRP (Fig. 1B).
Rats were divided equally into 3 groups (Fig. 2): Group 1: Control group; left colon was transected and end-to-end colonic anastomosis was performed, then hyperthermic (42℃) saline were perfused for 60 minutes. Group 2: Oxaliplatin alone group; left colon was transected and end-to-end colonic anastomosis was performed followed by HIPEC with oxaliplatin (77.5 mg/kg) at 42℃ and 5 mg/kg for 60 minutes. Group 3: Oxaliplatin and PRP group; left colon was transected, end-to-end colonic anastomosis was performed and PRP gel was applied to the anastomosis site, then HIPEC was performed with oxaliplatin (77.5 mg/kg) at 42℃ and 5 mg/kg for 60 minutes.
Ketamine hydrochloride (Ketalar, Park Devis, Istanbul, Turkey) at a dose of 50 mg/kg, and xylazine (Rompun, Bayer, Istanbul, Turkey) at a dose of 5 mg/kg were used for anesthesia. After skin shaving and skin preparation with 10% povidone iodine, a laparotomy was performed and the left colon was identified and transected. End-to-end anastomosis was performed with 5/0 polypropylene sutures (Prolene, Ethicon, Cincinnati, OH, USA). In group 3, PRP gel was applied all around the anastomosis line (Fig. 3). Inflow and outflow catheters were placed to the right supradiaphragmatic space and pelvis, respectively, then the abdomen was closed with separate 3/0 silk sutures. After closure of the abdomen oxaliplatin in 5% dextrose solution was perfused for 60 minutes by a roller pump in groups 2 and 3. The perfusate was heated by a heater plate and the temperature of the abdomen was monitored continuously with a digital thermometer. The animals were taken back to their cages and fed chow and water after the operation.
On the seventh day following surgery, the animals were reoperated on and both abdomen and colonic anastomosis were evaluated. The colon segment involving the anastomosis was removed and 2 rubber catheters were placed into the proximal and distal lumen of the excised segment. The catheters were fixed with 3/0 silk sutures and catheters were connected to a syringe pump and a manual sphygmomanometer to measure the anastomotic bursting pressure (ABP). The colon segment was insufflated with air by syringe pump and when the first air bubble was detected, the pressure value was recorded as ABP. After recording ABP, the colon segment was opened and divided into 2 pieces, one piece was to be used for histopathological analysis and therefore put into 10% formalin solution, and the other was stored at −20℃ for hydroxyproline assay. The rats were euthanized with a high dose of anesthetic agent.
All materials were fixed in formalin and embedded in paraffin. Haematoxylin-eosin stained sections were evaluated by one pathologist without knowledge of the group. An Olympus (BX51) light microscope was used. Verhofstad scale was used to evaluate the anastomotic healing by scoring the degrees of necrosis, neutrophils, macrophages, lymphocytes, edema, and mucosal epithelium [15].
Tissues were homogenized with liquid nitrogen and were soaked in a phosphate buffer solution (10 mM; pH, 7.2). Tissue samples were sonicated for 10 minutes at +4℃ with 220 V (METU electromechanical, Serial No. 30607, Berlin, Germany) before they were stored at −80℃ until assay. The homogenates, which were defrosted at room temperature, were later centrifuged at +4℃ for 5 minutes with 15,000 g. The supernatants were taken for hydroxyproline analysis. The concentrations of hydroxyproline in the sample were measured using a commercially available enzyme-linked immunosorbent Hydroxyproline Assay Kit (CEA621Ge, Cat. No.l180307228; Cloud-Clone Corp., TX, USA). This assay employs the competitive inhibition enzyme immunoassay technique. A monoclonal antibody specific to hydroxyproline was precoated onto a microplate. A competitive inhibition reaction was launched between biotin labeled hydroxyproline and unlabeled hydroxyproline (standards or samples) with the precoated antibody specific to hydroxyproline. After incubation the unbound conjugate was washed off. Next, avidin conjugated to horseradish peroxidase (HRP) was added to each microplate and incubated. The amount of bound HRP conjugate was in reverse proportion to the concentration of hydroxyproline in the sample. After addition of the substrate solution, the intensity of color developed was in reverse proportion to the concentration of hydroxyproline in the sample. The protein amount of each sample of the tissue homogenates was calculated with the Lowry method [16]. Tissue hydroxyproline values were expressed as ng per mg protein. All assays were conducted according to the manufacturer's instructions. The samples, which showed higher concentrations, were diluted and measured in duplicate.
Sample size was calculated by the statistical package (G*Power software) from previous experimental rat studies data on intestinal anastomosis healing with a power of 80% α-error and 5% β-error, and it is estimated that at least 10 samples were needed for each group. Numerical data were presented as mean ± standard deviation. One-way analysis of variance test was used for analyzing the differences between the mean values of the parameters (ABP, tissue hydroxyproline level, and histological scores) and either Tukey honestly significant difference or Tamhane post hoc tests were used to analyze the differences between the groups. A P-value below 0.05 was considered statistically significant.
All the animals survived until the end of the study. No wound complications, including wound infection, seroma, or wound dehiscence, occurred. No local or systemic complications related to PRP were observed. In group 3, it was observed that hyperthermic perfusion did not remove the PRP gel due to PRP gel adhering strongly to the anastomosis line.
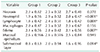
The ABP values of the groups are given in Table 1. In variant analysis, as expected, group 1 had higher ABP value than group 2 and 3 (P < 0.001 and P < 0.001, respectively). Group 2 had lower ABP than group 3 and the difference was significant (P < 0.001). These findings indicate that PRP gel application has a positive effect on ABP.
Table 1 shows the hydroxyproline levels of the groups. Group 2 had significantly lower hydroxyproline levels than groups 1 and 3 (P < 0.001). The difference between groups 1 and 3 was significant (P < 0.001). These data show that PRP application improved wound healing.
Verhofstad scale is a good guideline for evaluating anastomotic wound healing and is widely used in experimental studies [13151718]. In our study, histopathological analysis demonstrated that PRP application improved the anastomotic healing on a cellular basis by decreasing neutrophils and lymphocytes amounts, as well as degree of edema. Also, submucosal bridging was significantly better in group 1 and group 3 than in group 2 (Fig. 4) Detailed data analysis is shown in Tables 2 and 3.
Despite the survival advantages of CRS combined with HIPEC, this treatment modality has higher morbidity rates when compared with conventional surgical procedures. It is believed that morbidity would be caused by either major surgery or drug toxicity [19]. Anastomosis leakage can occur in up to 10%–25% of cases in those whom have undergone colorectal resections. For this reason, the majority of surgeons prefer to perform stomas (either ileostomy or colostomy) to prevent fecal peritonitis and its fatal results [20]. However, stoma closure requires additional surgical intervention, and reduced quality of life in patients with stomas has been reported [21]. Doud et al. [22] reported that stoma closure surgery is associated with a higher rate of morbidity and mortality for patients on whom HIPEC was performed.
PRP is an autologous concentrate that contains numerous growth factors, and several experimental studies have shown that PRP application improves wound healing as well as intestinal anastomosis healing [12132324]. In this experimental study, our data showed that PRP reduced tissue inflammation and increased hydroxyproline levels and ABP. These data suggest that PRP gel improves impaired anastomotic healing due to hyperthermic oxaliplatin perfusion. In light of these findings, we propose to use PRP gel reinforcement to colonic anastomosis to prevent stoma and stoma related morbidity and mortality by reducing anastomotic leakage rates. Our proposal has several strengths outlined as follows; PRP gel application is safe and has no reported adverse effects to date. It will also not cause any allergic events due to being autologous. PRP gel application is easy to prepare and use, can be obtained in about an hour in the operating room, and has a lower cost compared to other reinforcement materials [25]. Furthermore, we evaluated the anastomosis strength on postoperative day 7. Both experimental and clinical studies have shown that collagen lysis started in the first 3 to 5 days and anastomosis strength reached the lowest value on the seventh day, thus most of the anastomosis leakage occurred within those days [1226272829]. Median ABP value, as well as the median hydroxyproline level, was significantly higher in the PRP applied group (group 3) than in group 2. These data suggest that applying PRP gel has a positive impact on impaired anastomosis healing due to hyperthermic oxaliplatin perfusion.
This study has several limitations. First of all; this experiment was based on an animal model, thus the effects of this application on humans are unknown. Although there are numerous experimental studies suggesting favorable effects of PRP on intestinal anastomosis healing, interestingly, to date there are no clinical studies regarding this issue except one. Recently, Casella et al. [25] used PRP gel as a stapler line reinforcement material in patients who had received laparoscopic sleeve gastrectomy. They followed 20 patients and no leakage was observed during the 1-year follow-up period. Despite the small number of patients, the authors encourage us to use PRP on gastrointestinal anastomosis; of course, further clinical studies including a larger number of patients are needed. Secondly, the rats were reoperated at postoperative day 7 in our study. Therefore, any late complications could not be evaluated including abscess, ileus, etc., as well as postoperative mortality. Thirdly, and perhaps most importantly, concern of cancer progression has been considered by some authors [30]. PRP contains numerous growth factors, which improve wound healing, but could also increase the proliferation of tumor cells, and are unacceptable for cancer patients. However, the latter is not proven yet and still remains as a theory.
In conclusion, our data suggest the use of PRP gel on colonic anastomosis improves anastomotic wound healing in the cellular base and these findings will encourage the surgeons performing anastomosis without creation of stomas in patients who have received HIPEC with oxaliplatin. Further human studies are needed to support our suggestion.
Figures and Tables
Fig. 1
(A) Three layers were seen after first spin; plasma, buffy coat, and red blood cells layers. (B) Gel form of platelet-rich plasma.

Fig. 2
Study protocol. PRP, platelet-rich plasma; HIPEC, hyperthermic intraperitoneal chemotherapy. Group 1, control group; group 2, oxaliplatin alone group; group 3, oxaliplatin and platelet-rich plasma group.
Fig. 3
The gel form of platelet-rich plasma was applied around the colonic anastomosis. It adheres quickly to the anastomotic site and was not affected by hyperthermic oxaliplatin perfusion.
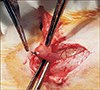
Fig. 4
(A) Group 1: There is marked submucosal bringing, less edema, and inflammation (H&E, ×40). (B) Group 2: There is huge necrotic exudate on the luminal surface and between the anastomosis sides (H&E, ×40). (C) Group 3: There is less inflammation, edema and more bringing (H&E, ×40). Group 1, control group; group 2, oxaliplatin alone group; group 3, oxaliplatin and platelet-rich plasma group.

Table 1
The mean values of anastomotic bursting pressure and hydroxyproline levels of the study groups

References
1. Aoyagi T, Terracina KP, Raza A, Takabe K. Current treatment options for colon cancer peritoneal carcinomatosis. World J Gastroenterol. 2014; 20:12493–12500.



2. Nadler A, McCart JA, Govindarajan A. Peritoneal carcinomatosis from colon cancer: a systematic review of the data for cytoreduction and intraperitoneal chemotherapy. Clin Colon Rectal Surg. 2015; 28:234–246.



3. Nagata H, Ishihara S, Hata K, Murono K, Kaneko M, Yasuda K, et al. Survival and prognostic factors for metachronous peritoneal metastasis in patients with colon cancer. Ann Surg Oncol. 2017; 24:1269–1280.

4. Bhatt A, Bhamre R, Rohila J, Kalikar V, Desouza A, Saklani A. Patients with extensive regional lymph node involvement (pN2) following potentially curative surgery for colorectal cancer are at increased risk for developing peritoneal metastases: a retrospective single-institution study. Colorectal Dis. 2019; 21:287–296.

5. Glehen O, Kwiatkowski F, Sugarbaker PH, Elias D, Levine EA, De Simone M, et al. Cytoreductive surgery combined with perioperative intraperitoneal chemotherapy for the management of peritoneal carcinomatosis from colorectal cancer: a multi-institutional study. J Clin Oncol. 2004; 22:3284–3292.


6. Verwaal VJ, van Ruth S, Witkamp A, Boot H, van Slooten G, Zoetmulder FA. Long-term survival of peritoneal carcinomatosis of colorectal origin. Ann Surg Oncol. 2005; 12:65–71.


7. Casado-Adam A, Alderman R, Stuart OA, Chang D, Sugarbaker PH. Gastrointestinal complications in 147 consecutive patients with peritoneal surface malignancy treated by cytoreductive surgery and perioperative intraperitoneal chemotherapy. Int J Surg Oncol. 2011; 2011:468698.

8. Hompes D, D’Hoore A, Van Cutsem E, Fieuws S, Ceelen W, Peeters M, et al. The treatment of peritoneal carcinomatosis of colorectal cancer with complete cytoreductive surgery and hyperthermic intraperitoneal peroperative chemotherapy (HIPEC) with oxaliplatin: a Belgian multicentre prospective phase II clinical study. Ann Surg Oncol. 2012; 19:2186–2194.


9. Gervais MK, Dube P, McConnell Y, Drolet P, Mitchell A, Sideris L. Cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy with oxaliplatin for peritoneal carcinomatosis arising from colorectal cancer. J Surg Oncol. 2013; 108:438–443.


10. Riss S, Chandrakumaran K, Dayal S, Cecil TD, Mohamed F, Moran BJ. Risk of definitive stoma after surgery for peritoneal malignancy in 958 patients: comparative study between complete cytoreductive surgery and maximal tumor debulking. Eur J Surg Oncol. 2015; 41:392–395.


11. Whealon MD, Gahagan JV, Sujatha-Bhaskar S, O’Leary MP, Selleck M, Dumitra S, et al. Is fecal diversion needed in pelvic anastomoses during hyperthermic intraperitoneal chemotherapy (HIPEC)? Ann Surg Oncol. 2017; 24:2122–2128.


12. Yamaguchi R, Terashima H, Yoneyama S, Tadano S, Ohkohchi N. Effects of platelet-rich plasma on intestinal anastomotic healing in rats: PRP concentration is a key factor. J Surg Res. 2012; 173:258–266.


13. Sozutek A, Colak T, Cetinkunar S, Reyhan E, Irkorucu O, Polat G, et al. The effect of platelet-rich-plasma on the healing of left colonic anastomosis in a rat model of intra-abdominal sepsis. J Invest Surg. 2016; 29:294–301.


14. Alves R, Grimalt R. A review of platelet-rich plasma: history, biology, mechanism of action, and classification. Skin Appendage Disord. 2018; 4:18–24.


15. Verhofstad MH, Lange WP, van der Laak JA, Verhofstad AA, Hendriks T. Microscopic analysis of anastomotic healing in the intestine of normal and diabetic rats. Dis Colon Rectum. 2001; 44:423–431.


16. Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951; 193:265–275.


17. Strebel K, Nielsen SR, Biagini M, Qvist N. Effect of Humira® on intestinal anastomotic response in rabbits. J Invest Surg. 2015; 28:167–172.


18. Aghayeva A, Benlice C, Bilgin IA, Atukeren P, Dogusoy G, Demir F, et al. The effects of hyperthermic intraperitoneal chemoperfusion on colonic anastomosis: an experimental study in a rat model. Tumori. 2017; 103:307–313.


19. von Breitenbuch P, Piso P, Schlitt HJ. Safety of rectum anastomosis after cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. J Surg Oncol. 2018; 118:551–556.
20. Deraco M, Glehen O, Helm CW, Morris DL, van der Speeten K. Cytoreductive surgery & perioperative chemotherapy for peritoneal surface malignancy: textbook and video atlas. Woodbury (CT): Cine-Med Publishing Inc.;2013.
21. Braumann C, Muller V, Knies M, Aufmesser B, Schwenk W, Koplin G. Quality of life and need for care in patients with an ostomy: a survey of 2647 patients of the Berlin OStomy-Study (BOSS). Langenbecks Arch Surg. 2016; 401:1191–1201.

22. Doud AN, Levine EA, Fino NF, Stewart JH, Shen P, Votanopoulos KI. Stoma creation and reversal after cytoreductive surgery with hyperthermic intraperitoneal chemotherapy. Ann Surg Oncol. 2016; 23:503–510.

23. Yol S, Tekin A, Yilmaz H, Kucukkartallar T, Esen H, Caglayan O, et al. Effects of platelet rich plasma on colonic anastomosis. J Surg Res. 2008; 146:190–194.


24. Esat Duymus M, Temel S, Ozer H, Kemal Urhan M, Kaya F, Aslan F, et al. Comparison of the effects of plateletrich plasma prepared in various forms on the healing of dermal wounds in rats. Wounds. 2016; 28:99–108.

25. Casella G, Soricelli E, Genco A, Ferrazza G, Basso N, Redler A. Use of platelet-rich plasma to reinforce the staple line during laparoscopic sleeve gastrectomy: feasibility study and preliminary outcome. J Laparoendosc Adv Surg Tech A. 2015; 25:222–227.


26. Hyman N, Manchester TL, Osler T, Burns B, Cataldo PA. Anastomotic leaks after intestinal anastomosis: it’s later than you think. Ann Surg. 2007; 245:254–258.


27. Zhou B, Ren J, Ding C, Wu Y, Chen J, Wang G, et al. Protection of colonic anastomosis with platelet-rich plasma gel in the open abdomen. Injury. 2014; 45:864–868.

28. Schiff A, Roy S, Pignot M, Ghosh SK, Fegelman EJ. Diagnosis and management of intraoperative colorectal anastomotic leaks: a global retrospective patient chart review study. Surg Res Pract. 2017; 2017:3852731.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download