Abstract
Purpose
The International Study Group on Pancreatic Fistula's definition of postoperative pancreatic fistula (POPF) has recently been updated. This study aimed to identify risk factors for POPF in patients having pancreaticoduodenectomy (PD) and to generate a nomogram to predict POPF.
Methods
Data on 298 patients who underwent PD from March 2012 to October 2017 was retrospectively reviewed and POPF statuses were redefined. A nomogram was constructed using data from 220 patients and validated using the remaining 78 patients. Independent risk factors for POPF were identified using univariate and multivariate analyses. A predictive nomogram was established based on the independent risk factors and was compared with existing models.
Results
Texture of the pancreas, size of the main pancreatic duct, portal vein invasion, and definitive pathology were the identified risk factors. The nomogram had a C-index of 0.793 and was internally validated. The nomogram performed better (C-index of 0.816) than the other most cited models (C-indexes of 0.728 and 0.735) in the validation cohort. In addition, the nomogram can assign patients into low- (less than 10%), intermediate- (10% to 30%), and high-risk (equal or higher than 30%) groups to facilitate personalized management.
Postoperative pancreatic fistula (POPF) is a frequent and intractable complication in patients who have had pancreatic resection. POPF is significantly associated with morbidity and can double the rate of mortality after surgery [1]. POPF rates after pancreatectomy and particularly after pancreaticoduodenectomy (PD) have been reported to range from 2% to 28% [23]. This large range is mainly due to the different methods of managing pancreatic remnant and the previously vague definition of POPF. To address this, the International Study Group on Pancreatic Fistula (ISGPF) suggested a POPF definition and grading system, which has been widely accepted since 2005 [4]. This system was recently updated by the ISGPF with clearer and more precise statements, based on the studies performed in the last decade. And POPF is now redefined as a drain output of any measurable volume of fluid with an amylase level >3 times the upper limit of institutional normal serum amylase activity, related to the clinically relevant condition caused by the pancreatic fistula directly. A novel concept of “biochemical leak” displaced the former grade A POPF under the circumstances, which is no longer considered a true fistula because of nonclinical importance [5]. These modifications, however, may lead to inconsistent diagnoses and classifications of POPF if different versions of the system are applied in clinical practice. Therefore, it is necessary to reassess historical data to understand the effect of the new classification system. In addition, a number of investigations were performed to identify risk factors for POPF using the original definition and classification [6]; here, we aim to reevaluate the risk factors for POPF using the updated system.
Based on risk factors, researchers have proposed several predictive models for the purposes of early identification and effective management of POPF in high-risk patients [7891011]. However, these models have not yet been widely accepted. One of the reasons for this is that Callery's model had an area under curve (AUC) of higher than 0.93 in the original cohort [7], but only had an AUC of 0.68 when used by another institute using the updated POPF definition [12]. In addition, some models, although they performed well, used extremely complicated formulas that lacked direct clinical significance [8]. Other models were based on relatively small cohorts [11]. Most importantly, accepted predictive models using the latest POPF definition have not yet been established and are urgently needed.
Nomograms are a proven, useful type of tool for predicting the risks of adverse events and likelihood of survival in various clinical scenarios [131415]. Compared with other decision aids including risk groupings, probability tables, artificial neural networks, and classification and regression tree analyses, nomograms provide evidence-based and highly accurate risk estimates for individualized decision-making [16]. Furthermore, nomograms are visual and are practical in clinical settings. In this study, we endeavored to determine the risk factors for POPF after PD using the new definition, and to generate a nomogram based on these risk factors for predicting risk of POPF in relevant patients.
The protocol of this study was reviewed by the Medical Ethics Committee of the First Affiliated Hospital, Zhejiang University School of Medicine (2019_K705), which waived the need for consent due to the retrospective nature of the study.
Patients who underwent PD for pancreatobiliary disorders at our center from March 2012 to June 2016 were retrospectively enrolled for development of the nomogram. Patients from August 2016 to October 2017 were enrolled with the same selection criteria and were analyzed as an independent internal validation set. Patients with incomplete information or uncertain POPF statuses were excluded.
The following data were extracted from the electronic medical database: sex, age, body mass index (BMI), morbidity, history of alcohol use and smoking, preoperative biliary drainage, biochemical results, and surgical information including but not limited to duration of surgery, estimated blood loss, texture of pancreas, size of the main pancreatic duct, and pancreaticojejunostomy (PJ) technique. To ensure accuracy, data were independently checked by 2 authors and judged by an experienced surgeon if the 2 authors did not agree.
Classic PD techniques were performed in all patients. In brief, the pancreatic head, neck, and uncinate process were removed, followed by removal of the duodenum, gallbladder, common bile duct, and distal part of the stomach. The duct of Wirsung (i.e., pancreatic duct) was measured and identified, along with the pancreas transected at the level of the left wall of the portal vein. Child's technique was used for gastrointestinal reconstruction. For a double-layer PJ, duct-to-mucosa or invagination anastomosis was used as previously described [17]. Before March 2015, the PJ technique was chosen randomly for the purpose of a clinical trial [17]. After this date, the PJ technique was chosen based on the characteristics of the pancreatic remnant and our experience. In patients with a firm pancreas and a dilated pancreatic duct (diameter no smaller than 3 mm), a duct-to-mucosa PJ was selected. Otherwise, an invagination PJ was preferred. If possible, an internal stent was inserted in the pancreatic duct. A sump drain-irrigation tube was routinely placed behind the bilioenteric anastomosis and beside the PJ anastomosis. A prophylactic jejunostomy feeding tube was placed only in patients with severe malnutrition. Fluid needs during the procedure were based on classical physiological parameters such as heart rate, blood pressure, and central venous pressure.
Previously, the postoperative care path mainly included prophylactic antibiotics, early enteral feeding or nutritional support, removal of the nasogastric and abdominal drains tube if meeting standards or agreements, respectively. Patients admitted after May 2014 were managed according to the predetermined protocol of enhanced-recovery after surgery [18]. Neither somatostatin nor octreotide was adopted prophylactically, but could be used if POPF was diagnosed. The volume and amylase level of drainage fluid were routinely monitored on postoperative day 3, 5, and 7 until drain removal or patient discharge. The drains were removed at the surgeon's discretion, when their total daily output was <30 mL, amylase level was below 3 times the serum upper level of normal value or appearance of the effluent was unalarming at that time. Closer observation, maintenance of the drain, or additional measures like therapeutic somatostatin, supplemental nutritional support or percutaneous drainage were taken, as appropriate, provided that a POPF had been diagnosed [5].
The primary outcome, POPF, was diagnosed according to the updated ISGPF definition and classification [5]. For reassessment of POPF with the new definition, we reviewed all the patient data regarding postoperative parameters relevant to POPF (e.g., amylase level in drainage fluid, duration of abdominal drain, interventional radiology procedures, reoperation, organ failure, length of hospital stay). Disagreements were resolved by discussion. Perioperative mortality was defined as any death occurring in the hospital or within 90 days of surgery. Readmission was counted when patients were readmitted within 90 days of discharge due to surgery-related complications. The severities of complications were graded using the Clavien-Dindo classification [19].
Data are presented as the median and interquartile range. Univariate and multivariate analyses were performed using IBM SPSS Statistics ver. 22.0 (IBM Co., Armonk, NY, USA). Categorical variables and continuous variables were compared using the chi-square test (Fisher exact test, when applicable) and Student t-test (or Mann-Whitney U-test), respectively. Univariate logistic regression analysis was used to assess the relationship between each variable and the development of POPF. All variables associated with POPF at a significant level then went through stepwise multivariate analysis. A nomogram was formulated based on the results of multivariate logistic regression analysis, using the rms package of R ver. 3.1.1 (R Foundation for Statistical Computing, Vienna, Austria; http://www.r-project.org/). The nomogram was created by proportionally converting each regression coefficient in the multivariate logistic regression to a 0- to 100-point scale. The effect of the variable with the highest β coefficient (absolute value) was assigned 100 points. Points are added across independent variables to derive total points, which are converted to predicted probabilities. The predictive performance of the nomogram was measured by concordance index (C-index) and the tool was calibrated with 500 bootstrap samples to decrease the overfit bias. A P-value less than 0.05 was considered to be statistically significant.
Of the 306 patients who were considered for enrollment in this study, 8 were excluded owing to incomplete clinical data. A total of 130 male and 168 female patients were included, with an average age of 63 years. During the study period, 220 patients met the inclusion criteria and were used for developing the nomogram (defined as the training cohort). Another 78 patients who met the inclusion criteria were used as the validation cohort. The ratio of patients in these groups was almost 3 to 1. The baseline characteristics of the patients did not differ significantly between the 2 groups (Table 1).
In all patients across both cohorts, the postoperative lengths of intensive care unit stay and hospital stay were 0 (range, 0–1) and 13.0 days (range, 8.0–20.0 days), respectively. The most frequent reasons for PD in these patients were pancreatic head cancer (46.6%), ampullary or duodenal cancer (17.8%), distal cholangiocarcinoma (12.4%), and pancreatic cystic neoplasms (16.1%) (Supplementary Tables 1, 2).
Univariate analysis revealed 5 factors that were significantly correlated with POPF. These were the texture of the pancreas, the diameter of the main pancreatic duct, PJ technique, portal vein invasion, and pathology type (Table 2). The incidence of POPF in patients with a soft or normal pancreas was higher than that in patients with a firm or fibrotic pancreas (37.9% vs. 9.4%, P < 0.001). Patients with small pancreatic main ducts (less than 3 mm) [20] were more likely to develop POPF than those with larger ducts (31.3% vs. 17.5%, P = 0.018). There was a lower POPF rate in patients with pancreatic head cancer or chronic pancreatitis than in patients with other diseases (16.3% vs. 28.4%, P = 0.032). A significant difference in POPF rate was also detected between the patients who underwent different PJ techniques, with a higher POPF rate in patients who had had invagination PJ (30.7% vs. 18.6%, P = 0.043).
Multivariate analysis showed that PJ technique did not independently predict POPF (Table 2). Of note, patients with a soft or normal pancreas (odds ratio [OR], 6.93; 95% confidence interval [CI], 3.13–15.32; P < 0.001) or with a small pancreatic duct (OR, 1.21; 95% CI, 1.00–1.47; P = 0.045) were at increased risk of developing POPF after PD. Those with portal vein invasion (OR, 0.34; 95% CI, 0.13–0.87; P = 0.025) or with pancreatic head cancer or pancreatitis (OR, 0.39; 95% CI, 0.18–0.83; P = 0.014) were less likely to develop POPF.
Based on the 4 independent risk factors, we constructed a nomogram for predicting the probability of POPF in patients who undergo PD (Fig. 1). The number of points assigned to each factor is weighted by the OR of that variable. The total number of points corresponds to the estimated probability that POPF will develop. For example, a patient who underwent PD because of pancreatic head cancer was found with portal vein invasion and had a soft pancreas, and the diameter of their main pancreatic duct was 3 mm. In this case, the total points would be around 115 and the corresponding probability that this patient will develop POPF is less than 10%.
In the training cohort, the C-index of the nomogram for POPF prediction was 0.793 (95% CI, 0.731–0.855). In addition, the calibration curve showed adequate consistency between predicted occurrence of POPF using the nomogram and the actually observed occurrence of POPF in the cohort (Fig. 2A).
In the validation cohort, the C-index of the nomogram for predicting POPF was 0.816 (95% CI, 0.684–0.949). The calibration plot showed good agreement between the predictions by the nomogram and actual observations (Fig. 2B).
To compare our nomogram with previously reported models, we tested 2 validated models (generated by Callery et al. [7] and Mungroop et al. [21]) using the validation cohort. The C-indexes of Callery's and Mungroop's models were 0.706 and 0.789, respectively (Fig. 3B, C). Our nomogram, with a C-index for this cohort of 0.816, performed better (Fig. 3A).
We identified 3 risk groups based on the risk distribution estimated by the nomogram using the whole cohort. The low-risk group (total points of less than 133.5, with a predicted POPF rate of less than 10%) had a predicted mean risk of 7.7%. The intermediate-risk group (total points of 133.5 to 178.25, with a predicted POPF rate of between 10% and 30%) had a predicted mean risk of 17.0%. The high-risk group (total points equal or more than 178.25, with a predicted POPF rate of equal to or higher than 30%) had a predicted mean risk of 46.7% (Table 3). Significant differences were noted when comparing the groups with each other about the observed POPF rates and predicted mean risks (P < 0.001).
There are significant variations in the incidences of adverse events related to POPF among these 3 different risk groups (Table 4).
Starting from most disease-relevant demographics and clinicopathological variables, we identified 4 independent risk factors for POPF. Most of these factors were well-known risk factors for POPF using the 2005 definition, except portal vein invasion, which was infrequently reported specifically as a risk factor for POPF after PD [222324]. In addition to the fact that many studies did not include portal vein invasion as a candidate risk factor [21], whether portal vein invasion was seen to be a risk factor may have been affected by the proportion of patients with malignancies and venous invasion in the cohort in question. For instance, in the study by Yamamoto et al. [10], incidences of pancreatic cancer (51.9%) and portal vein invasion (21.4%) were reported that were similar to those in our patient group (49.3% and 27.2%, respectively). Yamamoto et al. [10] also identified involved portal vein as an independent risk factor for POPF, although they used preoperative computed tomography evaluation compared with our postoperative pathological assessment.
Although some studies have revealed that BMI [2122] and intraoperative blood loss [725] were risk factors for POPF, we failed to confirm this in our patient group. Asian patients normally have a lower BMI compared with Western people [26]. In fact, only 14.8% of our participants had a BMI higher than 25, which could have led to an underestimate of the effect of a high BMI in the development of POPF. These variables are plausible as risk factors, the discrepancies between the different studies probably being due to instabilities in the models or differences in patient groups. Some previous investigations [272829] have reported a significant correlation between serum total bilirubin and POPF, although no such correlation was observed in the current study. The main reason for this is likely to be the different cutoff values used (e.g., 2.0, 3.5, or 4.6 mg/dL) to define elevation of the total bilirubin level. The reason for this potential relationship between total bilirubin and POPF is also poorly understood.
Among the independent risk factors, the texture of the pancreas has been the most definitive, being reported in nearly all similar studies. In the present study, this factor was seen to have the most potent influence on the development of POPF, with its OR of above 3. The size of the main pancreatic duct is also a well-accepted risk factor for POPF, and always has a relatively high OR [102123]. Since the PJ technique used can be largely dependent on the size of the main pancreatic duct, we speculate that the preference of surgeons for a certain PJ technique could affect the weight of the size of the pancreatic duct in predictive models. Therefore, the performance of a model in external cohorts may be substantially affected by different surgeons' preferences for PJ techniques.
The 4 factors finally included in the nomogram are easily obtained from patients. Three of them could be supposed on preoperative imaging data or recorded intraoperatively, leaving only pathology to be defined postoperatively. Although a definitive pathological diagnosis using paraffin sections of pancreatic lesions is typically reported in 1 week, which may delay the prediction of POPF development, it is possible to make a prediction with an accuracy of higher than 95% from the information gained during surgery in conjunction with pathological diagnosis using frozen sections [30]. Therefore, the final scores and risk of developing POPF can be predicted in a timely manner rather than being delayed until several days after the operation.
By using the nomogram, we were able to classify patients who underwent PD into 3 distinct risk groups. Clinical outcomes related to POPF also worsen in an escalating fashion as points accumulate. Patients require more invasive interventions and suffer from more severe morbidity with the increased risk groups. To avoid inappropriate treatment, specific management strategies can be established according to the level of risk. We suggest early removal of abdominal drains and an enhanced-recovery pathway in the low-risk group, even if a slight increase in amylase level is observed. Close observation and timely intensive care (such as being monitored in intensive care units) may be planned postoperatively in the intermediate- and high-risk groups, which accounted for 35.9% and 30.2% of all patients in this study, respectively. Especially for the high-risk patients, a small increase in amylase level in the drainage fluid warrants serious consideration and possible interventions. Moreover, the frequency of amylase level tests may depend on not only the actual situation, but the risk level of the patient.
The current study has several limitations. The retrospective nature of the assessment of the presence of POPF using the updated criteria may have led to biases due to inaccurate or incomplete records. We excluded patients with uncertain POPF statuses; however, this action in itself is likely to have introduced selection bias. A prospective study is therefore warranted, and we are preparing to test the nomogram in a prospective cohort. Another limitation was that this nomogram requires postoperative variables like texture of the pancreas and especially definitive pathology, which is likely to lead to delayed or not very precise prediction, and inevitably, poor clinical impact despite the above mentioned. In addition, although nearly 300 patients were included in this study, this is a relatively small number compared with some previous reports. For instance, one study of an alternative fistula risk score included 1924 patients in the model design cohort and another 926 patients in the external validation cohort [21]. Braga's study used 2 cohorts containing 700 patients [28], and Callery's study included a total of 445 patients [7]. Moreover, as the analysis and suggestions were based on data from a single institution, further studies are needed to validate the results externally and to confirm the value in predicting POPF in order to provide enough data to support potential routine applications in clinical decision-making.
In conclusion, we created a nomogram to predict the development of POPF in patients who have undergone PD, based on the 2016 definition of POPF. The nomogram provides a convenient, visual tool for surgeons to predict the risk of POPF in individual patients, facilitating postoperative management after PD surgery. The reliability and effectiveness of the predictive model should be tested in more clinical trials.
Figures and Tables
Fig. 1
Nomogram to predict the probability that postoperative pancreatic fistula will develop in patients who have undergone pancreaticoduodenectomy.

Fig. 2
Calibration curves comparing predicted and actual probabilities of postoperative pancreatic fistula in the training cohort (A) and in the validation cohort (B).
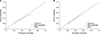
Fig. 3
Comparison of the accuracy of the prediction of postoperative pancreatic fistula development in the validation cohort between the nomogram (A) and the conventional models, Callery's model [7] (B) and Mungroop's model [21] (C). AUC, area under curve.

ACKNOWLEDGEMENTS
This study was financially supported by National High Technology Research and Development Program of China (No. S2015AA020405), National Natural Science Foundation of China (No. 81672337), Key Program of the National Natural Science Foundation of China (No. 81530079), Key research and development Project of Zhejiang Province (No.2015C03044), Zhejiang Provincial Program for the Cultivation of High-level Innovative Health Talents, Zhejiang Provincial Key Innovation Team of Pancreatic Cancer Diagnosis & Treatment (No. 2013TD06) and China Scholarship Council (No. 201706320169). The authors thank Jian-Feng Wang for his assistance with the information collection. And Cheng-Xiang Guo especially expresses his gratitude and blessings to Jiaqi Zhao, whose radio shows have given him continuous confidence and motivation in his college years.
Notes
References
1. Vin Y, Sima CS, Getrajdman GI, Brown KT, Covey A, Brennan MF, et al. Management and outcomes of postpancreatectomy fistula, leak, and abscess: results of 908 patients resected at a single institution between 2000 and 2005. J Am Coll Surg. 2008; 207:490–498.

2. Allen PJ, Gonen M, Brennan MF, Bucknor AA, Robinson LM, Pappas MM, et al. Pasireotide for postoperative pancreatic fistula. N Engl J Med. 2014; 370:2014–2022.


3. Ramacciato G, Mercantini P, Petrucciani N, Nigri GR, Kazemi A, Muroni M, et al. Risk factors of pancreatic fistula after pancreaticoduodenectomy: a collective review. Am Surg. 2011; 77:257–269.

4. Bassi C, Dervenis C, Butturini G, Fingerhut A, Yeo C, Izbicki J, et al. Postoperative pancreatic fistula: an international study group (ISGPF) definition. Surgery. 2005; 138:8–13.

5. Bassi C, Marchegiani G, Dervenis C, Sarr M, Abu Hilal M, Adham M, et al. The 2016 update of the International Study Group (ISGPS) definition and grading of postoperative pancreatic fistula: 11 years after. Surgery. 2017; 161:584–591.

6. Vallance AE, Young AL, Macutkiewicz C, Roberts KJ, Smith AM. Calculating the risk of a pancreatic fistula after a pancreaticoduodenectomy: a systematic review. HPB (Oxford). 2015; 17:1040–1048.



7. Callery MP, Pratt WB, Kent TS, Chaikof EL, Vollmer CM Jr. A prospectively validated clinical risk score accurately predicts pancreatic fistula after pancreatoduodenectomy. J Am Coll Surg. 2013; 216:1–14.


8. Roberts KJ, Hodson J, Mehrzad H, Marudanayagam R, Sutcliffe RP, Muiesan P, et al. A preoperative predictive score of pancreatic fistula following pancreatoduodenectomy. HPB (Oxford). 2014; 16:620–628.


9. Ven Fong Z, Correa-Gallego C, Ferrone CR, Veillette GR, Warshaw AL, Lillemoe KD, et al. Early drain removal--the middle ground between the drain versus no drain debate in patients undergoing pancreaticoduodenectomy: a prospective validation study. Ann Surg. 2015; 262:378–383.

10. Yamamoto Y, Sakamoto Y, Nara S, Esaki M, Shimada K, Kosuge T. A preoperative predictive scoring system for postoperative pancreatic fistula after pancreaticoduodenectomy. World J Surg. 2011; 35:2747–2755.


11. Gaujoux S, Cortes A, Couvelard A, Noullet S, Clavel L, Rebours V, et al. Fatty pancreas and increased body mass index are risk factors of pancreatic fistula after pancreaticoduodenectomy. Surgery. 2010; 148:15–23.


12. Miao Y, Lu Z, Zhu Q, Yin J, Zhang K, Dai C, et al. Risk prediction for pancreatic fistula and risk-stratified strategy for prophylactic intra-abdominal drainage after Whipple’s procedure. Pancreatology. 2017; 3 Supplement:S94.

13. Gemenetzis G, Bagante F, Griffin JF, Rezaee N, Javed AA, Manos LL, et al. Neutrophil-to-lymphocyte ratio is a predictive marker for invasive malignancy in intraductal papillary mucinous neoplasms of the pancreas. Ann Surg. 2017; 266:339–345.


14. Jang JY, Park T, Lee S, Kim Y, Lee SY, Kim SW, et al. Proposed nomogram predicting the individual risk of malignancy in the patients with branch duct type intraductal papillary mucinous neoplasms of the pancreas. Ann Surg. 2017; 266:1062–1068.

15. de Castro SM, Biere SS, Lagarde SM, Busch OR, van Gulik TM, Gouma DJ. Validation of a nomogram for predicting survival after resection for adenocarcinoma of the pancreas. Br J Surg. 2009; 96:417–423.

16. Shariat SF, Capitanio U, Jeldres C, Karakiewicz PI. Can nomograms be superior to other prediction tools. BJU Int. 2009; 103:492–495.

17. Bai X, Zhang Q, Gao S, Lou J, Li G, Zhang Y, et al. Duct-to-mucosa vs invagination for pancreaticojejunostomy after pancreaticoduodenectomy: a prospective, randomized controlled trial from a single surgeon. J Am Coll Surg. 2016; 222:10–18.


18. Bai X, Zhang X, Lu F, Li G, Gao S, Lou J, et al. The implementation of an enhanced recovery after surgery (ERAS) program following pancreatic surgery in an academic medical center of China. Pancreatology. 2016; 16:665–670.


19. Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004; 240:205–213.


20. Shukla PJ, Barreto SG, Fingerhut A, Bassi C, Buchler MW, Dervenis C, et al. Toward improving uniformity and standardization in the reporting of pancreatic anastomoses: a new classification system by the International Study Group of Pancreatic Surgery (ISGPS). Surgery. 2010; 147:144–153.


21. Mungroop TH, van Rijssen LB, van Klaveren D, Smits FJ, van Woerden V, Linnemann RJ, et al. Alternative fistula risk score for pancreatoduodenectomy (a-FRS): design and international external validation. Ann Surg. 2019; 269:937–943.

22. Hu BY, Wan T, Zhang WZ, Dong JH. Risk factors for postoperative pancreatic fistula: analysis of 539 successive cases of pancreaticoduodenectomy. World J Gastroenterol. 2016; 22:7797–7805.



23. Liu QY, Zhang WZ, Xia HT, Leng JJ, Wan T, Liang B, et al. Analysis of risk factors for postoperative pancreatic fistula following pancreaticoduodenectomy. World J Gastroenterol. 2014; 20:17491–17497.



24. Akamatsu N, Sugawara Y, Komagome M, Shin N, Cho N, Ishida T, et al. Risk factors for postoperative pancreatic fistula after pancreaticoduodenectomy: the significance of the ratio of the main pancreatic duct to the pancreas body as a predictor of leakage. J Hepatobiliary Pancreat Sci. 2010; 17:322–328.


25. Fu SJ, Shen SL, Li SQ, Hu WJ, Hua YP, Kuang M, et al. Risk factors and outcomes of postoperative pancreatic fistula after pancreatico-duodenectomy: an audit of 532 consecutive cases. BMC Surg. 2015; 15:34.



26. NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: a pooled analysis of 2416 population-based measurement studies in 128•9 million children, adolescents, and adults. Lancet. 2017; 390:2627–2642.


27. Rungsakulkij N, Mingphruedhi S, Tangtawee P, Krutsri C, Muangkaew P, Suragul W, et al. Risk factors for pancreatic fistula following pancreaticoduodenectomy: a retrospective study in a Thai tertiary center. World J Gastrointest Surg. 2017; 9:270–280.



28. Braga M, Capretti G, Pecorelli N, Balzano G, Doglioni C, Ariotti R, et al. A prognostic score to predict major complications after pancreaticoduodenectomy. Ann Surg. 2011; 254:702–707.


29. Kimura W, Miyata H, Gotoh M, Hirai I, Kenjo A, Kitagawa Y, et al. A pancreaticoduodenectomy risk model derived from 8575 cases from a national single-race population (Japanese) using a web-based data entry system: the 30-day and in-hospital mortality rates for pancreaticoduodenectomy. Ann Surg. 2014; 259:773–780.

SUPPLEMENTARY MATERIAL
Supplementary Tables 1–2 can be found via https://www.astr.or.kr/src/sm/astr-98-72-s001.pdf.




 PDF
PDF ePub
ePub Citation
Citation Print
Print







 XML Download
XML Download