Abstract
Purpose
Nipple-sparing mastectomy (NSM) has become increasingly popular due to improved cosmesis without compromising oncologic safety. Radial and inframammary incisions are usually used to achieve NSM, with periareolar incisions usually being avoided because of the risk to nipple-areola complex viability. In an attempt to maximize esthetic effects, we performed NSM through periareolar incision with immediate reconstruction. We report our initial experience.
Methods
This case series consisted of all consecutive patients (n = 34) who underwent NSM through a periareolar incision in our institution between August 2017 and December 2018. All patients underwent NSM through periareolar incision followed by immediate reconstruction with an implant or deep inferior epigastric perforator flap. Patient demographics, tumor and treatment characteristics, and short-term postoperative outcomes were reviewed.
Results
The mean patient age was 46.74 ± 6.69 years (range, 38–62 years), and the mean operation time was 96.68 ± 28.00 minutes. Indications included in situ cancer in 12 cases and invasive cancer in 22 cases. There was 1 major complication (postoperative hematoma) requiring operative reintervention. No other complications including fistula, implant exposure, or reconstruction failure was observed. At the time of writing, no case of local recurrence has been observed.
Since its initial description in the 1960s by Freeman [12], nipple-sparing mastectomy (NSM) without indication for breast-conserving surgery has become a standard procedure with curative intent for breast cancer [3]. Recent studies report that NSM can be used in all cases in which total mastectomy is indicated when histological analyses of intraoperative frozen sections from sub-nipple-areola complex (NAC) tissues are negative [4]. NSM has demonstrated improved cosmetic outcome without compromising oncologic safety [56].
Various NSM approaches have been applied, and there are various incisions associated with these approaches, including inframammary, radial, and periareolar incisions, which have been extensively described in the literature [3789]. Inframammary incisions leave a discreet scar, but access to the breast and axilla is difficult. Radial incisions provide enough exposure and easy access to the axilla but are considered “radical” because of the amount of exposure [379].
Periareolar incisions have not been routinely used because they are technically difficult and thought to be associated with increased risk of ischemia and necrosis of the NAC. However, periareolar incisions can produce the best esthetic results because the incision scar remains well hidden within the periphery of the NAC. Recently, the NSM approaches with a periareolar incision have been reported to produce successful outcomes, including no serious NAC complications [810].
In an attempt to maximize esthetic effects, we also used this periareolar incision approach. We performed NSM through periareolar incision and 1-stage immediate reconstruction. Herein we present our first results with this approach.
This case series consisted of 34 consecutive patients who underwent NSM through only periareolar incision, followed by immediate reconstruction with an implant or deep inferior epigastric perforator (DIEP) flap, at our institution between August 2017 and November 2018. Patient demographics, tumor and treatment characteristics, and short-term postoperative outcomes were reviewed.
All patients agreed to go through with the procedure after being informed about the potential benefits and risks. A single breast surgeon performed all NSM operations. The medical records and pathology data of all the patients were reviewed for this case series, and this retrospective study was approved by Ewha Womans University Medical Center Institutional Review Board (IRB No. 2019-07-012). Informed consent was waived due to the retrospective nature of this study.
Under general anesthesia, the patient is placed in supine position with the ipsilateral arm abducted 90 degrees. In all cases, the surgeon made a semicircular incision along the NAC (Fig. 1). NSM was performed with the standard technique, layer by layer, in accordance with oncological criteria. Dissection is carried on to reach the clavicle superiorly, inframammary fold inferiorly, the edge of the sternum medially, and the anterior border of the latissimus dorsi laterally.
Intraoperative biopsy samples from sub-NAC tissues were sent for frozen section. If the frozen biopsy result is tumor-positive, these cases were excluded from this study. Sentinel lymph node (SLN) evaluation was done through the same periareolar incision or a different axillary incision. Reconstructions with implants or DIEP flaps were performed by a plastic surgeon immediately after completion of the mastectomy by the breast surgeon.
To evaluate surgical outcomes, we evaluated the following surgical variables: operation time, mastectomy specimen weight, implant and DIEP volume, surgical wound infection, seroma, dehiscence, fistula, skin necrosis, NAC necrosis (mild, moderate, and total), implant exposure, and reconstruction failure. Postradiation contractures were quantified with the Baker scale (grade I, the breast is normally soft and appears natural in size and shape; grade II, the breast is a little firm, but appears normal; grade III, the breast is firm and appears abnormal; grade IV, the breast is hard, painful to the touch, and appears abnormal).
The mean age and body mass index were 46.74 ± 6.69 years (range, 38–62 years) and 22.68 ± 2.71 kg/m2 (range, 18.5–27.6 kg/m2), respectively. The operations indications were distributed as follows: luminal (n = 16), HER2 (n = 3), triple-negative (n = 3), and carcinoma in situ (n = 12). Bilateral mastectomy was performed in 3 cases due to the bilateral breast cancer. Mean operation time for mastectomy was 96.68 ± 28.00 minutes. Six cases of SLN evaluation were done through the same periareolar incision, and 28 cases were performed through additional axilla incision. The mean weight of the mastectomy specimens was 277.84 ± 85.81 g, and the mean volume and weight of the implants and DIEP flaps were 273.97 ± 86.50 mL and 357.67 ± 146.41 g, respectively (Table 1).
One case of hematoma occurred, and this was treated with a hematoma evacuation operation. One wound infection and 1 minor NAC problem was observed. No other complications including fistula, implant exposure, or reconstruction failure was observed. Ten patients (29.4%) received adjuvant chemotherapy, and 2 patients (5.9%) received adjuvant radiotherapy. Nineteen patients (55.9%) received hormonal therapy. During a median follow-up period (18.2 months), no grade III or IV Baker scale contractures were observed. At the time of writing, no case of local recurrence had been observed (Table 2).
In this study, all NSMs were performed by the same surgeon, potentially minimizing case-by-case variation in our initial report. NSM through periareolar incision with immediate reconstruction was revealed to be technically feasible and to allow easy access to the mammary parenchyma. The approach was performed successfully with low rates of complications.
Various incision types have been used to remove glandular tissue and prepare flaps, depending on tumor location and breast type. Periareolar incisions have not been usually performed because of the risk to NAC viability [11121314]. Our operative method emphasizes the careful preservation of the periareolar dermis to maximize NAC viability after NSM. Recently, the approach of NSM with a periareolar incision and 2-stage reconstruction has also been shown to produce successful outcomes [8]. However, 2-stage reconstruction could be inconvenient for the patients. Furthermore, the team who reported outcomes of the periareolar incision and 2-stage reconstruction used a semicircular incision below the NAC with a 3- to 4-cm lateral radial elongation, which is not a true periareolar incision and would have sacrificed cosmetic considerations.
We performed 1-stage immediate breast reconstruction in which the plastic surgeon placed a breast implant or DIEP flap immediately after the breast surgeon removed any malignant breast tissue. One-stage immediate reconstruction has several advantages [1516]. One-stage surgery lowers risk, decreases costs, minimizes patient discomfort and maximizes patient satisfaction. Tissue expansion after mastectomy in 2-stage surgery can be avoided. Reduced scarring and better cosmetic results can be expected also. Furthermore, faster recovery and return to active lifestyle can be expected.
We performed NSM through only periareolar incision without any elongation, which maximized cosmesis. Patients with large NACs are ideal candidates for NSM through a single periareolar incision. For those with very small areolae and large breast volume, medial and/or lateral extensions may be needed to perform the mastectomy meticulously and for easier extraction of the surgical specimen [810]. We used a lateral periareolar semicircular incision for easy access to the axilla (Fig. 1). However, for easy access to the locations of tumor, a superior or inferior semicircular incision also can be considered. In Table 3, we compared several periareolar approaches including our method.
Of relevance to operation procedure, preservation of fatty tissue around the axillary tail and fibrous tissue of the inframammary fold is also important to maintain the original breast contour after reconstruction. The fasciae of the serratus anterior and rectus abdominis should also be preserved during NSM to avoid implant dislocation. In 6 cases of ductal carcinoma in situ, SLN biopsies were successfully performed through a single periareolar incision. All of these surgical techniques maximize the cosmesis.
There was 1 major complication (postoperative hematoma) requiring reoperation (bleeding control was performed in pectoralis muscle with general anesthesia). One minor NAC problem (delayed wound healing) case was occurred and cured with steri-strip apply. One case of wound infection was treated incision and drainage with local anesthesia and antibiotics treatment. No other complications including fistula, implant exposure, or reconstruction failure was observed.
Overall, the esthetic outcomes were satisfactory (Fig. 2) and comparable to previous study results [1718]. Periareolar incisions without any elongation produce well-hidden incision scars within the periphery of the NAC (Fig. 2). Radiotherapy was administered after NSM according to international mastectomy guidelines, and only low level of capsular contracture (Baker grade I) was observed.
The oncological safety of NSM has been subject to debate [561718]. So far, no prospective randomized study has been conducted to demonstrate the efficacy of the technique. In the present study, no recurrence was observed after 18.2 months of median follow-up, although the sample was small and the follow-up period was short.
In summary, our initial report shows that NSM with immediate reconstruction may safely be performed through periareolar incision. This method can maximizes cosmesis and may be a good surgical option for NSM.
Figures and Tables
Fig. 1
Nipple-sparing mastectomy incision. The round line indicates the breast parenchyme to be resected and the red semicircular line shows the place to make incision.
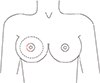
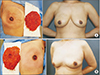
Fig. 2
Intraoperative and late postoperative view. Periareolar incisions produced well-hidden incision scars within the periphery of the nipple-areola complex. (A) A 51-year-old female with invasive ductal carcinoma of right breast. We performed right nipple-sparing mastectomy with periareolar approach. Resected mastectomy volume was 293 g. (B) A 47-year-old female with intraductal carcinoma in situ of left breast. We performed left nipple-sparing mastectomy with periareolar approach. Resected mastectomy volume was 334 g.
References
1. Freeman BS. Subcutaneous mastectomy for benign breast lesions with immediate or delayed prosthetic replacement. Plast Reconstr Surg Transplant Bull. 1962; 30:676–682.


2. Freeman BS. Complications of subcutaneous mastectomy with prosthetic replacement, immediate or delayed. South Med J. 1967; 60:1277–1280.


3. Rawlani V, Fiuk J, Johnson SA, Buck DW 2nd, Hirsch E, Hansen N, et al. The effect of incision choice on outcomes of nipple-sparing mastectomy reconstruction. Can J Plast Surg. 2011; 19:129–133.



4. Paepke S, Schmid R, Fleckner S, Paepke D, Niemeyer M, Schmalfeldt B, et al. Subcutaneous mastectomy with conservation of the nipple-areola skin: broadening the indications. Ann Surg. 2009; 250:288–292.

5. Gerber B, Krause A, Dieterich M, Kundt G, Reimer T. The oncological safety of skin sparing mastectomy with conservation of the nipple-areola complex and autologous reconstruction: an extended follow-up study. Ann Surg. 2009; 249:461–468.


6. Orzalesi L, Casella D, Santi C, Cecconi L, Murgo R, Rinaldi S, et al. Nipple sparing mastectomy: surgical and oncological outcomes from a national multicentric registry with 913 patients (1006 cases) over a six year period. Breast. 2016; 25:75–81.


7. Blechman KM, Karp NS, Levovitz C, Guth AA, Axelrod DM, Shapiro RL, et al. The lateral inframammary fold incision for nipple-sparing mastectomy: outcomes from over 50 immediate implant-based breast reconstructions. Breast J. 2013; 19:31–40.


8. Cavalcante FP, Lima MVA. Nipple-sparing mastectomy with periareolar incision and two-stage reconstruction: initial analysis of 31 cases. Breast J. 2018; 24:940–943.


9. Frey JD, Salibian AA, Levine JP, Karp NS, Choi M. Incision choices in nipple-sparing mastectomy: a comparative analysis of outcomes and evolution of a clinical algorithm. Plast Reconstr Surg. 2018; 142:826e–835e.
10. El Hage Chehade H, Headon H, Wazir U, Carmaichael AR, Choy C, Kasem A, et al. Nipple-sparing mastectomy using a hemi-periareolar incision with or without minimal medial-lateral extensions; clinical outcome and patient satisfaction: a single centre prospective observational study. Am J Surg. 2017; 213:1116–1124.


11. Vaughn CJ, Peled AW, Esserman LJ, Foster RD. Feasibility of performing total skin-sparing mastectomy in patients with prior circumareolar mastopexy or reduction mammoplasty incisions. Ann Plast Surg. 2013; 06. 19. DOI: 10.1097/SAP.0b013e3182977904. [Epub].

12. Algaithy ZK, Petit JY, Lohsiriwat V, Maisonneuve P, Rey PC, Baros N, et al. Nipple sparing mastectomy: can we predict the factors predisposing to necrosis? Eur J Surg Oncol. 2012; 38:125–129.


13. Munhoz AM, Aldrighi CM, Montag E, Arruda EG, Aldrighi JM, Gemperli R, et al. Clinical outcomes following nipple-areola-sparing mastectomy with immediate implant-based breast reconstruction: a 12-year experience with an analysis of patient and breast-related factors for complications. Breast Cancer Res Treat. 2013; 140:545–555.


14. Regolo L, Ballardini B, Gallarotti E, Scoccia E, Zanini V. Nipple sparing mastectomy: an innovative skin incision for an alternative approach. Breast. 2008; 17:8–11.


15. Pek WS, Tan BK, Ru Ng YY, Kiak Mien Tan V, Rasheed MZ, Kiat Tee Tan B, et al. Immediate breast reconstruction following nipple-sparing mastectomy in an Asian population: aesthetic outcomes and mitigating nipple-areolar complex necrosis. Arch Plast Surg. 2018; 45:229–238.



16. Gougoutas AJ, Anderson BO. One-stage versus two-stage breast reconstruction: prudence in surgical decision-making. Lancet Oncol. 2017; 18:166–167.






 PDF
PDF ePub
ePub Citation
Citation Print
Print






 XML Download
XML Download