Abstract
Purpose
Hepatorenal syndrome (HRS) is a fatal complication in patients with end-stage liver disease awaiting liver transplantation (LT). HRS often develops in patients with high model for end-stage liver disease (MELD) score. This study investigated the outcomes of peritransplant management of HRS in a high-volume LT center in Korea for 2 years.
Methods
A total of 157 recipients that deceased donor liver transplantation (DDLT) from January 2017 to December 2018 were included. In-hospital mortality (IHM) was analyzed in relation to pre- and posttransplant application of renal replacement therapy (RRT).
Results
Primary diagnoses for DDLT were alcoholic liver disease (n = 61), HBV-associated liver cirrhosis (n = 48), retransplantation for chronic graft failure (n = 24), and others (n = 24). Mean MELD score was 34.6 ± 6.2 with 72 patients at Korean Network for Organ Sharing MELD status 2 (45.9%), 43 at status 3 (27.4%), 36 at status 4 (22.9%), and 6 at status 5 (3.8%). Pretransplant RRT was performed in 16 patients (10.2%) that did not show IHM. Posttransplant RRT was performed in 69 patients (44.0%), for whom IHM incidence was 15.9%. In 53 patients that had undergone de novo posttransplant RRT, IHM incidence increased to 20.8%. IHM in the 88 patients not requiring RRT was 2.3%.
Conclusion
The majority of adult DDLT recipients in Korean MELD score-based allocation system have very high MELD scores, which is often associated with HRS. Pretransplant RRT appears to improve posttransplant survival outcomes. We thereby recommend that, if indicated, pretransplant RRT be performed while awaiting DDLT.
Optimized allocation of deceased donor organs is a grave concern for patients on the waiting list for liver transplantation (LT). In Korea, a nationwide allocation system for deceased donor liver grafts was started in February 2000 after establishment of the Korean Network for Organ Sharing (KONOS) modeled after the United Network for Organ Sharing [12]. This system utilized the Child-Turcotte-Pugh (CTP) score-based allocation system, similar to that used in the United States, before the adoption of the MELD score-based allocation system. The annual number of deceased organ donors in Korea is much lower than in many Western countries, and a serious liver organ shortage led to high waiting-list mortality rates. As such, the Korean MELD score-based allocation system was adopted in June 2016 to optimize the use of deceased donor liver organs [345].
This new allocation system has been successfully practiced for more than 3 years, to date. However, the number of deceased donors in Korea did not increase over this period. As a result, the majority of deceased donor liver organs were allocated to patients with very high MELD scores [6]. A MELD score close to 40 indicates that overt renal dysfunction has already developed, as this score is associated with a significant rise in serum creatinine. The proportion of patients requiring peritransplant renal replacement therapy (RRT) for renal replacement or support was markedly increased after implementation of the new allocation system. Peritransplant management of hepatorenal syndrome (HRS) is now more essential than ever before [7].
This study investigates the status and outcomes of peritransplant management of HRS in an LT center performing a high-volume deceased donor liver transplantations (DDLTs).
This study was a retrospective analysis using single-institution DDLT data from the Asan Medical Center. The first 6 months after adoption of the MELD score-based allocation system were not included due to the high number of late retransplantation cases during this period [6]. Posttransplant observation for 6 months or longer is necessary to determine the outcomes of patients that received peritransplant RRT. Thus, the study period for patient selection was the 2 years from January 2017 to December 2018. In-hospital mortality was compared in relation to pre- and posttransplant RRT. Institutional Review Board of Asan Medical Center approved the protocol of this study (2014-0831).
The Korean MELD score-based allocation system uses the following original calculation formula: [9.57 × loge (creatinine, mg/dL) + 3.78 × loge (total bilirubin, mg/dL) + 11.2 × loge (INR) + 6.43]. There are 5 categories of MELD score status: status 1 (acute liver failure and early graft failure), status 2 (MELD score 38–40, equivalent to old KONOS status 2A), status 3 (MELD score 31–37), status 4 (MELD score 21–30), and status 5 (MELD score ≤20). Patients with hepatocellular carcinoma (HCC) within the Milan criteria receive an additional 4 or 5 points if their MELD score is less than or equal to 20. Pediatric end-stage liver disease scoring is used for patients up to 12 years of age [6].
HRS often develops in patients with acute-on-chronic liver failure and the clinical sequence of late graft failure after LT is similar to that of acute-on-chronic liver failure. As such, the selected study patients were adult DDLT recipients diagnosed with chronic liver diseases or late graft failure. KONOS status 1 patients were excluded because they tend to show different clinical courses compared to other status patients. A total of 157 patients over the 2-year study period (January 2017–December 2018) were selected and followed up through July 2019 or until patient death.
A cross-sectional study was performed with a 2-month observation period from October 2018 to November 2018 to evaluate the sequential changes of MELD scores in individual patients. We included all patients enrolled with a KONOS status 2 or 3 already on the waiting list during this period. Sequential changes of MELD scores in 22 patients were recorded until DDLT or patient death without LT.
HRS is a reversible, functional defect in renal function seen in advanced liver disease and is characterized by functional renal vasoconstriction with minimal renal histologic abnormalities leading to a severe reduction in glomerular filtration rate. There are 2 types of HRS. HRS type 1 is defined by at least a 2-fold increase in serum creatinine (reflecting a 50% reduction in creatinine clearance) to a level greater than 2.5 mg/dL over a period of fewer than 2 weeks, and it is the most common form of acute kidney injury (AKI) associated with liver cirrhosis and is associated with a very poor prognosis without treatment. HRS type 2 is defined by less severe renal impairment than that observed in type 1 disease and is usually associated with refractory ascites [89].
These concepts of HRS have been recently changed to severe forms of AKI. AKI is generally defined as an increase in serum creatinine by at least 0.3 mg/dL, at 1.5-fold over baseline, or urine output less than 0.5 mg/kg/hour for more than 6 hours [10]; presentation with RIFLE (risk, injury, failure, loss, and end-stage) criteria [11]; AKI Network criteria [12]; Kidney Disease Improving Global Outcomes criteria [13]; or International Club of Ascites-AKI criteria [14].
Pretransplant renal dysfunction is initially treated with supportive care including albumin and terlipressin [1516]. If supportive care is not effective, RRT is initiated in the form of continuous veno-venous hemodialysis (CVVHD) or continuous veno-venous hemodiafiltration (CVVHDF) [17]. Conventional intermittent hemodialysis is usually not chosen unless a CVVHD/CVVHDF machine is unavailable.
Our institutional indications for RRT in patients awaiting DDLT are renal replacement for overt manifestation of HRS and renal support for impending overt HRS with pulmonary edema or pneumonia, or impending overt HRS with hepatic encephalopathy or uremic syndrome. These patients also often require ventilator support.
Incidence variables were compared using the chi-square test and Fisher exact test. Patient survival curves were estimated using the Kaplan-Meier method and compared using the log-rank test. A P-value less than 0.05 was considered statistically significant. Statistical analyses were performed using IBM SPSS Statistics ver. 22.0 (IBM Co., Armonk, NY, USA).
Patient profiles are summarized in Table 1. Primary diagnoses for DDLT were alcoholic liver disease (n = 61), HBV-associated liver cirrhosis (n = 48), retransplantation for chronic graft failure (n = 24) and others (n = 24). The mean MELD score was 34.6 ± 6.2, and the MELD score categories presented in the study groups were KONOS status 2 (n = 72; 45.9%), status 3 (n = 43; 27.4%), status 4 (n = 36; 22.9%), and status 5 (n = 6; 3.8%). Distribution of detailed MELD scores is depicted in Fig. 1. The majority of patients at statuses 4 and 5 received liver grafts from marginal donors.
The distribution of serum creatinine levels at last MELD score calculation was creatinine ≤1.5 mg/dL in 70 patients, creatinine 1.51–2.0 mg/dL in 31 patients, creatinine 2.01–2.5 mg/dL in 16 patients, and creatinine >2.5 mg/dL in 40 patients.
During a 2-month observation period, 22 patients at KONOS status 2 or 3 were newly enrolled on the waiting list. Of them, 15 patients underwent DDLT, and remaining 7 died before LT. Sequential changes of MELD scores in individual patients are depicted in Fig. 2.
Initial MELD scores in KONOS status 2 patients were 40 (n = 4), 39 (n = 2) with none presenting 38. None of these patients presented with a decrease in MELD score. Four of them underwent DDLT, and the other 2 died while awaiting LT.
Initial MELD scores in remaining 16 KONOS status 3 patients ranged from 31 to 37. Of them, 4 patients were upgraded to KONOS status 2, and 3 of them underwent DDLT, and one died. Seven patients in KONOS status 3 underwent DDLT, and 5 died while awaiting LT.
Pretransplant RRT was performed for 16 patients (10.2%). Pretransplant ventilator support was performed for 34 patients (21.7%). Concurrent pretransplant application of RRT and ventilator support was performed for 9 patients (5.7%). The other 116 patients (73.9%) received neither RRT nor ventilator support while waiting for DDLT (Fig. 3).
After DDLT, 69 patients (44.0%) underwent RRT. A total of 53 patients (33.8%) presented with severe de novo renal dysfunction requiring posttransplant RRT. Of these, 25 patients received intraoperative RRT that was continued after DDLT operation; these patients might have indicated for pretransplant RRT, but did not receive it due to a shortage of RRT machines.
A total of 14 patients died during a median follow-up period of 514 days, with in-hospital mortality in 13 patients and one patient who died after discharge from the hospital. Overall 1-year and 2-year patient survival rates were 90.9% and 90.9%, respectively.
In the 16 recipients that received pretransplant RRT, all were successfully discharged from the hospital after DDLT, with no in-hospital mortality. Conversely, 13 of the 141 patients that did not receive pretransplant RRT died during hospitalization, yielding an in-hospital mortality rate of 9.2% (P = 0.36). Their overall 1-year patient survival rate was 89.9% and was not statistically different from that of the pretransplant RRT group (P = 0.192) (Fig. 4). The primary causes of patient death in 13 in-hospital mortality cases were multiorgan failure in 9, pneumonia with ischemic bowel necrosis or necrotizing pancreatitis in 3, and bleeding in 1.
Among the 69 recipients that received posttransplant RRT, 11 patients died during hospitalization, yielding an in-hospital mortality rate of 15.9%; this also was not significantly different from that of the pretransplant RRT group (P = 0.115).
However, compared with pretransplant RRT, 11 of the 53 patients receiving de novo posttransplant RRT died, yielding an in-hospital mortality rate of 20.8%; this was marginally greater than that for pretransplant RRT cases (P = 0.056). The in-hospital mortality rate in the 88 patients not requiring peritransplant RRT was 2.3% (Fig. 5).
The demand for LT in Korea has been high for a long period due to the high prevalence of HBV-associated liver cirrhosis and a high incidence of HCC [4520]. The annual number of deceased organ donors temporarily exceeded 10 per million in 2016 [3], but has since decreased, likely because of certain medical and social issues, including the Life Insurance Decision Act with regulations regarding the termination of life-sustaining treatment. Annual numbers for deceased organ donors and DDLT in Korea were 573 and 508 in 2016, 515 and 450 in 2017, and 449 and 369 in 2018, respectively.
In recent years, the total annual number of LT in Korea has remained largely unchanged, suggesting a reciprocal relationship between the case numbers of DDLT and living LDLT [4521]. Currently, critically ill patients, defined as those with very high MELD scores, are more likely to receive DDLT than before, thus they have usually waited for a significant period of time after enrollment on the KONOS waiting list. This allocation is associated with a waiting period of unpredictable duration. Thus, urgent LDLT is also performed, particularly in critically ill patients with progressive hepatic encephalopathy.
One of the primary reasons why the liver allocation system was changed from the old CTP score-based system to the new MELD score-based system was that relisting was not permitted after passing the 2-week priority allocation in patients classified as old KONOS status 1 or 2A. In this CTP score-based allocation system, patients who did not receive DDLT within 2 weeks could not indicate for priority allocation again, meaning if they did not receive LT during that time, most patients, save those who received LDLT, died. This was conducted to keep the pool size of critically ill patients on the waiting list from expanding. With new MELD score-based allocation, the pool size was gradually expanded as patients were permitted to wait for prolonged periods, either until receiving DDLT or dying without LT. Since the number of deceased organ donors cannot meet the DDLT demand for critically ill patients, priority allocation is given only to the patients with the highest KONOS status.
Before adoption of MELD score-based liver allocation system, we expected that the new KONOS statuses 2 and 3 would make up approximately half of DDLT cases, with a considerable proportion of liver grafts allocated to status 4 patients with MELD score between 21 and 30. However, in reality, the proportion of status 2 and 3 patients was much greater than anticipated. Recent decreases in the number of deceased donors induced further expansion of the critically ill waiting-list pool. We presume that this deleterious cycle is responsible for the increased number of DDLT recipients presenting with HRS.
We previously analyzed the changes in volumes and outcomes of DDLT for 1 year before and after introduction of MELD score in our institution. The number of patients with MELD score ≥ 31 was significantly increased after adoption of MELD score, but 3-month patient mortality rate was not significantly deteriorated (11.5% vs. 9.9%, P = 0.91) [6]. We presumed that our posttransplant management protocols for patients with very high MELD score including RRT and ventilator support have been already established through long-term experience with high number of such patients, thus potential detrimental effects from sudden increase of such patients might be diluted.
Theoretically, nearly all patients at KONOS status 2 have HRS and are thus indicated for RRT as a bridge therapy while awaiting LT. However, the waiting period for DDLT is highly variable, sometimes lasting several weeks, and the number of available CVVHD/CVVHDF machines at a given institution is often limited, thus complicating decisions over which patients start RRT and when to start RRT [7]. In clinical practice, renal dysfunction in critically ill patients awaiting LT tends not to improve even with RRT, and RRT often continues during and after LT operation.
These limitations in RRT have necessitated a prudent method to decide whether and when to perform RRT. Combined worsening of other organs function is a more serious condition than the solitary manifestation of HRS because functional failure of multiple organs is associated with marked increases in patient mortality. As such, when lung problems such as pulmonary edema or pneumonia or neurological problems such as hepatic encephalopathy or uremic syndrome are combined with HRS, this results in a priority indication of RRT for renal replacement or support. These conditions often require ventilator support in addition to RRT.
The cross-sectional analysis over 2-month observation period performed in this study revealed 3 discrete clinical pathways to liver graft allocation. The first pathway was sustained KONOS status 2 or high status 3, after which they underwent DDLT or died within 1 or 2 weeks. The second pathway involved a gradual increase in MELD score from low status 3 to high status 3 or status 2 over 1 to 3 weeks. The third pathway involved a fluctuation of MELD scores around low status 3 for several weeks, in which the probability of DDLT was low unless the MELD score gradually increased.
A MELD score spike is defined as a MELD score increase of at least 30% over a 7-day period. This usually accompanies overt manifestations of HRS and significantly increases the risk of early mortality [22]. A MELD score spike is a critical indication for pretransplant RRT as a bridge therapy to LT. Of the 3 described pathways, the first is eligible indication for pretransplant RRT due to high risk of early mortality. Many patients originally in the second pathway are indicated for pretransplant RRT due to MELD score spike. If pulmonary or neurological complications are combined, even patients in the third pathway are also indicated for pretransplant RRT.
CVVHD is a common form of RRT used for end-stage liver disease patients with renal dysfunction. Such patients usually have hyperdynamic circulation with relative hypotension owing to splanchnic vasodilatation and increased endotoxin and nitric oxide levels. Intermittent hemodialysis often induces hypotension, and patients with raised intracranial pressure can be deteriorated during hemodialysis, rendering them unsuitable for HRS treatment in liver cirrhosis. CVVHD offers improved hemodynamic stability without increasing intracranial pressure and is regarded as the safest support modality for hemodynamically unstable or encephalopathic patients requiring hemodialysis. Cardiovascular and intracranial stability in CVVHD occurs as a result of removal of cardiac depressant or proinflammatory factors during CVVHD [23]. For patients with hepatic encephalopathy, CVVHD removes toxins implicated in the pathogenesis of this condition, including ammonia and middle molecules. However, CVVHD does not remove lipid-soluble toxins, hepatotoxins, ammonia, and certain potential neurotoxins. Some studies have suggested that CVVHDF might be a better option for patients with worsening hepatic encephalopathy and poor renal function [24]. Conversely, however, another study reported that there was no difference between outcomes following CVVHD and CVVHDF [25].
Several studies have demonstrated that the survival outcomes of patients that underwent pretransplant RRT were comparable to those that did not require it [2627]. The survival outcomes of patients that underwent posttransplant RRT, however, are inferior to those of patients that did not need it. The results of the present study also suggest a beneficial effect of pretransplant RRT. These findings strongly recommend the provision of pretransplant RRT while awaiting DDLT, if it is indicated. The timely application of RRT before LT resulted in favorable posttransplant outcomes comparable to those for patients not requiring RRT. It has also been reported that pretransplant RRT in patients with HRS has little or no negative impact on patient or graft survival after LT [25].
In a meta-analysis with 9 studies comprising 3,941 patients [26], 1-year survival rates for patients receiving pretransplant CVVHD were similar to those with normal renal function during the pretransplantation period. However, patients with posttransplant renal failure requiring RRT demonstrated increased rates of 1-year mortality. Patients who developed de novo renal dysfunction after LT requiring RRT had lower 1-year survival rates than those of patients who started RRT before LT. This large-scale meta-analysis using patient population presenting with liver cirrhosis and renal failure suggests beneficial effects of pretransplant RRT as evidenced by improved patient survival rates compared to renal failure patients that did not receive pretransplant RRT.
The decision to start RRT before or during LT operation is based on multiple factors including general patient condition, metabolic and water-electrolyte disturbances, and anticipated hemodynamic instability during recipient hepatectomy. Indications for intraoperative RRT include patients at high risk for brain or pulmonary edema and severe intraoperative lactic acidosis or aggravation thereof. Intraoperative CVVHD can promote hemodynamic stability during the anhepatic and graft reperfusion stages of LT [2829]. Outcomes of patients requiring intraoperative RRT, however, are rarely reported. In a study with 41 cases of intraoperative RRT, the 1-year patient survival rate was 75.6%, but the 1-year incidence of chronic kidney disease was also high as 62.1%. While the safety and feasibility of intraoperative RRT have been proven, intraoperative RRT is limited to critically ill patients at risk for hemodynamic and metabolic instability during LT operation [29].
This study has several limitations of note. First, it was a single-center study, which could potentially introduce selection bias. We thereby recommend multicenter or nationwide follow-up studies to validate our results. Second, it was a retrospective study and the indications for pretransplant RRT were not clearly defined. Waiting-list mortality and posttransplant renal function were also not assessed in this study.
In conclusion, HRS is a frequent and fatal complication in patients with end-stage liver disease awaiting LT. Since only patients with very high MELD scores are allocated DDLT due to serious organ shortage in Korea, the majority of adult DDLT candidates also can suffer from HRS. Data from this study suggest that pretransplant RRT appears to improve posttransplant patient survival outcomes. Thus, we recommend that, if indicated, pretransplant RRT be performed while awaiting DDLT.
Figures and Tables
Fig. 1
Distribution of model for end-stage liver disease (MELD) scores in 157 patients who underwent deceased donor liver transplantation.
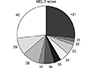
Fig. 2
Sequential changes of end-stage liver disease (MELD) scores in 22 patients on the liver transplantation waiting list. Squares indicate deceased donor liver transplantation (DDLT); circles indicate waiting-list mortality; and triangles indicate patient mortality after waiting for more than 1 month. Multiple lines indicate patient number.

Fig. 3
Distribution of patients that underwent pretransplant renal replacement therapy (RRT), ventilator support, and posttransplant RRT.
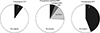
Fig. 4
Comparison of posttransplant patient survival curves according to pretransplant renal replacement therapy (RRT).

ACKNOWLEDGEMENTS
This study was supported by the Research Fund of the Asan Medical Center Organ Transplantation Center
References
1. Bollinger RR, Cho WH. Organ allocation for transplantation in the USA and Korea: the changing roles of equity and utility. Yonsei Med J. 2004; 45:1035–1042.


2. Hwang S, Ahn CS, Kim KH, Moon DB, Ha TY, Song GW, et al. Survival rates among patients awaiting deceased donor liver transplants at a single high-volume Korean center. Transplant Proc. 2013; 45:2995–2996.


3. Min SI, Ahn C, Han DJ, Kim SI, Chung SY, Lee SK, et al. To achieve national self-sufficiency: recent progresses in deceased donation in Korea. Transplantation. 2015; 99:765–770.

4. Lee SG, Moon DB, Hwang S, Ahn CS, Kim KH, Song GW, et al. Liver transplantation in Korea: past, present, and future. Transplant Proc. 2015; 47:705–708.


5. Jung BH, Hwang S, Song GW, Jung DH, Ha TY, Park GC, et al. Updated status of deceased-donor liver graft allocation for high-urgency adult patients in a Korean high-volume liver transplantation center. Transplant Proc. 2015; 47:580–583.


6. Ha SM, Hwang S, Song GW, Ahn CS, Moon DB, Ha TY, et al. Successful introduction of Model for End-stage Liver Disease scoring in deceased donor liver transplantation in Korea: analysis of first 1 year experience at a high-volume transplantation center. Ann Hepatobiliary Pancreat Surg. 2017; 21:199–204.



7. Francoz C, Durand F, Kahn JA, Genyk YS, Nadim MK. Hepatorenal syndrome. Clin J Am Soc Nephrol. 2019; 14:774–781.



8. Salerno F, Gerbes A, Gines P, Wong F, Arroyo V. Diagnosis, prevention and treatment of hepatorenal syndrome in cirrhosis. Gut. 2007; 56:1310–1318.



9. Alessandria C, Ozdogan O, Guevara M, Restuccia T, Jimenez W, Arroyo V, et al. MELD score and clinical type predict prognosis in hepatorenal syndrome: relevance to liver transplantation. Hepatology. 2005; 41:1282–1289.


10. Garcia-Tsao G, Parikh CR, Viola A. Acute kidney injury in cirrhosis. Hepatology. 2008; 48:2064–2077.


11. Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P. Acute Dialysis Quality Initiative workgroup. Acute renal failure - definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care. 2004; 8:R204–R212.
12. Mehta RL, Kellum JA, Shah SV, Molitoris BA, Ronco C, Warnock DG, et al. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007; 11:R31.

13. Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract. 2012; 120:c179–c184.

14. Angeli P, Gines P, Wong F, Bernardi M, Boyer TD, Gerbes A, et al. Diagnosis and management of acute kidney injury in patients with cirrhosis: revised consensus recommendations of the International Club of Ascites. Gut. 2015; 64:531–537.


15. Sagi SV, Mittal S, Kasturi KS, Sood GK. Terlipressin therapy for reversal of type 1 hepatorenal syndrome: a meta-analysis of randomized controlled trials. J Gastroenterol Hepatol. 2010; 25:880–885.


16. Boyer TD, Sanyal AJ, Garcia-Tsao G, Blei A, Carl D, Bexon AS, et al. Predictors of response to terlipressin plus albumin in hepatorenal syndrome (HRS) type 1: relationship of serum creatinine to hemodynamics. J Hepatol. 2011; 55:315–321.


17. AlEnezi F, Alhazzani W, Ma J, Alanazi S, Salib M, Attia M, et al. Continuous venovenous hemofiltration versus continuous venovenous hemodiafiltration in critically ill patients: a retrospective cohort study from a Canadian tertiary centre. Can Respir J. 2014; 21:176–180.



18. Agopian VG, Dhillon A, Baber J, Kaldas FM, Zarrinpar A, Farmer DG, et al. Liver transplantation in recipients receiving renal replacement therapy: outcomes analysis and the role of intraoperative hemodialysis. Am J Transplant. 2014; 14:1638–1647.


19. Matuszkiewicz-Rowinska J, Wieliczko M, MaXMLLink_XYZyszko J. Renal replacement therapy before, during, and after orthotopic liver transplantation. Ann Transplant. 2013; 18:248–255.


20. Korean Association for the Study of the Liver. KASL clinical practice guidelines: management of chronic hepatitis B. Clin Mol Hepatol. 2016; 22:18–75.


21. Moon DB, Lee SG, Hwang S, Kim KH, Ahn CS, Ha TY, et al. Toward more than 400 liver transplantations a year at a single center. Transplant Proc. 2013; 45:1937–1941.


22. Massie AB, Luo X, Alejo JL, Poon AK, Cameron AM, Segev DL. Higher mortality in registrants with sudden model for end-stage liver disease increase: disadvantaged by the current allocation policy. Liver Transpl. 2015; 21:683–689.


23. Kumar A, Thota V, Dee L, Olson J, Uretz E, Parrillo JE. Tumor necrosis factor alpha and interleukin 1beta are responsible for in vitro myocardial cell depression induced by human septic shock serum. J Exp Med. 1996; 183:949–958.



24. Jones CH, Richardson D, Goutcher E, Newstead CG, Will EJ, Cohen AT, et al. Continuous venovenous high-flux dialysis in multiorgan failure: a 5-year single-center experience. Am J Kidney Dis. 1998; 31:227–233.

25. Cordoba J, Blei AT, Mujais S. Determinants of ammonia clearance by hemodialysis. Artif Organs. 1996; 20:800–803.


26. Thorat A, Jeng LB. Management of renal dysfunction in patients with liver cirrhosis: role of pretransplantation hemodialysis and outcomes after liver transplantation. Semin Vasc Surg. 2016; 29:227–235.


27. Gonwa TA, Klintmalm GB, Levy M, Jennings LS, Goldstein RM, Husberg BS. Impact of pretransplant renal function on survival after liver transplantation. Transplantation. 1995; 59:361–365.






 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download