Abstract
Background and Purpose
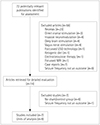
Methods
Results
Figures and Tables
Fig. 2
Forest plot of the included studies pooled together using a random-effects model for assessing the change in seizure frequency (A). Randomized controlled trials are indicated by the first author and year of publication. The size of each box is proportional to the weight of the corresponding study in the analysis, and the lines represent its 95% CI. Each open diamond represents the pooled relative risk, and its width represents the corresponding 95% CI. The sensitivity analysis excluded the study of Seynaeve et al.20 (B). *Sham vs. round coil, †Sham vs. figure of 8 coil, ‡1 Hz vs. placebo, §0.33 Hz vs. placebo. rTMS: repetitive transcranial magnetic stimulation.

Fig. 3
Forest plot of the included studies pooled together using a random-effects model for assessing the change in interictal epileptiform discharges. Randomized controlled trials are indicated by the first author and year of publication. The size of each box is proportional to the weight of the corresponding study in the analysis, and the lines represent its 95% CI. Each open diamond represents the pooled relative risk, and its width represents the corresponding 95% CI. rTMS: repetitive transcranial magnetic stimulation.

Fig. 4
Bubble plot of the effect of the duration of the follow-up period (adjusted for type of coil, duration of active treatment, and stimulation frequency) on the mean difference in seizure frequency. The studies/units of analysis are depicted by circles along the line of the meta-regression. The Y-axis represents the treatment effect and the X-axis represents the covariates used in the meta-regression analysis. The size of each symbol is inversely proportional to the variance of the estimated treatment effect.

Table 1
Characteristics of the included studies

CSSRS: Columbia Suicide Severity Rating Scale, DRE: drug-resistant epilepsy, ED: epileptiform discharge, QOLIE-31: Quality of Life in Epilepsy Inventory-31, RCT: randomized controlled trial, RMT: resting motor threshold, rTMS: repetitive transcranial magnetic stimulation, SCL-90: Symptom Checklist-90.
Table 3
Risk-of-bias assessment

B1: Random sequence generation (selection bias). B2: Allocation concealment (selection bias). B3: Blinding of participants and personnel (performance bias). B4: Blinding of outcome assessment (detection bias). B5: Incomplete outcome data (attrition bias). B6: Selective reporting (reporting bias). B7: Other type of bias.
Notes
Author Contributions
Conceptualization: Biswa Ranjan Mishra, Rituparna Maiti.
Data curation: Archana Mishra, Monalisa Jena, Rituparna Maiti, Anand Srinivasan.
Formal analysis: Archana Mishra, Anand Srinivasan.
Methodology: Archana Mishra, Monalisa Jena, Rituparna Maiti.
Project administration: Rituparna Maiti.
Resources: Anand Srinivasan.
Supervision: Rituparna Maiti.
Writing—original draft: Archana Mishra, Monalisa Jena, Rituparna Maiti.
Writing—review & editing: Anand Srinivasan.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download