Abstract
Background and Purpose
Methods
Results
Figures and Tables
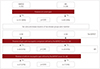 | Fig. 1Flow chart of the pain investigation in this study. BPI: brief pain inventory, NMOSD: neuromyelitis optica spectrum disorder, MS: multiple sclerosis. |
Acknowledgements
Notes
Author Contributions
Conceptualization: Jae-Won Hyun, Ho Jin Kim.
Data curation: Jae-Won Hyun, Hyunmin Jang, JaeBin Yu, Na Young Park, Su-Hyun Kim, So-Young Huh, Woojun Kim, Min Su Park, Jeeyoung Oh, Kee Duk Park, Ho Jin Kim.
Formal analysis: Jae-Won Hyun.
Funding acquisition: Jae-Won Hyun, Ho Jin Kim.
Investigation: Jae-Won Hyun, Hyunmin Jang, JaeBin Yu, Na Young Park, Su-Hyun Kim, So-Young Huh, Woojun Kim, Min Su Park, Jeeyoung Oh, Kee Duk Park, Ho Jin Kim.
Methodology: Jae-Won Hyun, Ho Jin Kim.
Project administration: Jae-Won Hyun.
Resources: Jae-Won Hyun, Hyunmin Jang, JaeBin Yu, Na Young Park, Su-Hyun Kim, So-Young Huh, Woojun Kim, Min Su Park, Jeeyoung Oh, Kee Duk Park, Ho Jin Kim.
Software: Jae-Won Hyun.
Supervision: Ho Jin Kim.
Validation: Jae-Won Hyun, Hyunmin Jang, JaeBin Yu, Na Young Park, Su-Hyun Kim, So-Young Huh, Woojun Kim, Min Su Park, Jeeyoung Oh, Kee Duk Park, Ho Jin Kim.
Visualization: Jae-Won Hyun.
Writing—original draft: Jae-Won Hyun, Ho Jin Kim.
Writing—review & editing: Jae-Won Hyun, Hyunmin Jang, JaeBin Yu, Na Young Park, Su-Hyun Kim, So-Young Huh, Woojun Kim, Min Su Park, Jeeyoung Oh, Kee Duk Park, Ho Jin Kim.
Conflicts of Interest Jang H, Yu J, Park NY, Huh, Kim W, Park MS, Oh J, Park KD report no financial disclosures. Hyun JW has received a grant from the National Research Foundation of Korea. Kim SH has lectured, consulted, and received honoraria from Bayer Schering Pharma, Biogen, Genzyme, Merck Serono, and UCB and received a grant from the National Research Foundation of Korea. Kim HJ has lectured, consulted, and received honoraria from Bayer Schering Pharma, Biogen, Celltrion, Eisai, HanAll BioPharma, Merck Serono, Novartis, Sanofi Genzyme, Teva-Handok, and UCB; received a grant from the National Research Foundation of Korea; and accepted research funding from Sanofi Genzyme, Teva-Handok, and UCB; serves on a steering committee for MedImmune/VielaBio; is a co-editor for the Multiple Sclerosis Journal-Experimental, Translational, and Clinical, and an associated editor for the Journal of Clinical Neurology.




 PDF
PDF ePub
ePub Citation
Citation Print
Print






 XML Download
XML Download