This article has been
cited by other articles in ScienceCentral.
Abstract
Background and Purpose
Intraoperative monitoring of the motor pathways is a routine procedure for ensuring the integrity of descending motor tracts during spinal surgery. Intraoperative motor evoked potential improvement (MEPI) may be associated with a better postsurgical outcome in cervical spondylotic myelopathy (CSM). To compare the efficacy of two cortical stimulation parameters in eliciting MEPI intraoperatively during CSM surgery.
Methods
We studied 69 patients who underwent decompression surgery for CSM over a 9-month period using either 5 (Group 1) or 9 (Group 2) stimuli. MEPI was defined as the increase in the amplitude of MEPs from baseline at the end of CSM surgery just prior to skin closure.
Results
An MEPI of 100% from baseline was observed in 10 patients (53%) in Group 1 and 36 patients (72%) in Group 2. Comparisons of the baseline mean MEP amplitudes of muscles bilaterally between Groups 1 and 2 did not reveal any significant differences. Supramaximal stimulation showed that a significantly higher mean intensity was required for Group 1 than for Group 2.
Conclusions
MEPI is observed in a much larger proportion of cervical decompression surgery cases than previously thought. Intraoperative MEPI with longer-train cortical stimulation may reflect adequacy of decompression and provide additional guidance for the surgical procedure.
Keywords: cervical spondylosis, cervical myelopathy, motor evoked potential, cortical stimulation, intraoperative monitoring, surgery
INTRODUCTION
Cervical spondylotic myelopathy (CSM) is a chronic progressive disease resulting from degeneration of the spinal cord and impingement on the nerve root by osteocartilaginous elements.
1 These lesions cause significant morbidity in patients, including gait instability, sensorimotor limb deficits, and bladder and bowel dysfunction. Many patients with CSM are treated surgically with the aim of preventing further neurological deterioration or achieving some functional recovery.
12 There is evidence from previous studies that the improvement of motor function after surgical decompression in CSM patients occurs via synaptic changes and dendritic sprouting in the cortical and spinal cord neuron pools.
345
In CSM, compression of descending corticospinal tracts results in desynchronization of I-wave volleys evoked by single-pulse transcranial magnetic stimulation (TMS) of the primary motor cortex. A prospective study of 141 CSM patients demonstrated a strong correlation of MRI findings with central motor conduction times in terms of sensitivity and specificity.
6 Another prospective study of 241 patients found that TMS parameters had a sensitivity and specificity of 98% for mild cord compression, suggesting that TMS can be employed as a screening tool in CSM before MRI.
7
Intraoperative monitoring (IOM) of the motor pathways is a routine procedure for ensuring the integrity of descending motor tracts during spinal surgery. Our previous studies found that multipulse cortical stimulation probably evoked not only corticospinal tracts but also reticulospinal and vestibulospinal tracts contralaterally, ipsilaterally, and transcallosally.
8910 In severe CSM, the mechanical compression of descending motor tracts contributes to neurological deficits, and may be reflected in abnormalities of the intraoperative motor evoked potentials (MEPs).
In two studies that have addressed IOM in CSM surgery, the intraoperative improvement of MEPs was referred to as a ‘positive change’ and ‘MEP signal improvement’.
1112 The first of these studies involved 59 patients, of which 21 (36%) showed improvement in MEPs, which correlated with a better prognosis at up to 6 months. In the second study involving 29 patients, 11 (38%) had a better outcome only at 1 month postoperatively. Both of these studies showed low rates of MEP improvement (MEPI) intraoperatively, and the specific stimulation positions and parameters were not described. Here we report our experience of MEPI during IOM in CSM surgery using two different cortical stimulation paradigms.
METHODS
We studied 69 patients who underwent decompression surgery for CSM over a 9-month period. None of the included patients had stroke, epilepsy, pacemaker insertion, other causes of spinal cord disease, or neuromuscular disorders. The Institutional Ethics Committee had previously approved the study protocols (IRB No. 2019/2347).
All of the CSM patients were diagnosed based on clinical complaints of neck pain, sensory symptoms and signs, motor weakness, presence of spastic gait, and MRI findings. The patients underwent decompression surgery from an anterior or posterior approach, and the protocol included laminectomy and instrumentation. We excluded patients with confounding factors such as neuropathy, myopathy, stroke, or previous spinal surgery.
The IOM protocol using total intravenous anesthesia and the cortical stimulation methodology have been published previously.
1314 Anesthesia was induced by administering propofol at 1–2 mg/kg and fentanyl at 2 mcg/kg. A single intravenous dose of 0.8 mg/kg atracurium was used to facilitate endotracheal intubation. The subsequent use of neuromuscular blocking agents was avoided. Anesthesia was maintained using 10 mg/kg propofol for the first 10 minutes, 8 mg/kg for the next 10 minutes, and 6 mg/kg for the remainder of the operation. Oxygen was administered at 50% in air. Remifentanil at a dosage of 0.03–0.1 mcg/kg/minute and morphine were titrated as needed for analgesia. Electrocardiography, pulse oximetry, capnography, and direct radial artery pressures were monitored. A bispectral-index monitor was used in all of the patients, with the depth of anesthesia maintained at about 40 on the index. All patients were kept normothermic with a warming blanket, and normotensive anesthesia was maintained throughout the operation.
After approximately 45 minutes postinduction, a fourt-witch assessment was performed using a nerve stimulator (NS242, Fisher & Paykel, Berkshire, UK) on the median nerve at the wrist. Cortical stimulation was commenced only when the amplitude of the fourth twitch of the abductor pollicis muscle was visibly similar to the first, which suggested that the effects of neuromuscular blocking agents had subsided.
Cortical stimulation was delivered by corkscrew electrodes at C1–C2 according to the international 10–20 system. A cross-scalp stimulating configuration was employed in which C1 was the active stimulating electrode position for left cortical stimulation while C2 was used for right cortical stimulation. The stimulation for both groups consisted of a train of square-wave stimuli 75 µs in duration delivered over a period of 11 ms, corresponding to a frequency of 750 Hz. The level of neuromuscular blockade was standardized to a train-of-four ratio of >0.75. The stimulator output was increased in steps of 50 V until a morphologically reproducible MEP with the largest amplitude was elicited. The intensity was then increased to 10% above this threshold intensity to obtain a supramaximal MEP response recorded with 13-mm disposable subdermal needles (Cadwell Industries, Kennewick, WA, USA) in the deltoid, flexor carpi radialis, abductor digiti minimi, tibialis anterior, and abductor hallucis bilaterally. The amplifier filter was set to a passband from 10 to 3,000 Hz, and the input impedances of the stimulating and recording electrodes remained below 5 kΩ. MEPs were recorded by means of a Medtronic NIMEclipse E4 system (Medtronic Xomed, Jacksonville, FL, USA). Peak-to-peak amplitudes and onset latencies was measured for MEP responses in each limb elicited by contralateral cortical stimulation. Ten consecutive supramaximal MEPs obtained after exposing the skin and muscle layers were averaged to obtain a final mean amplitude and latency as a baseline.
A standard left Smith-Robinson anterior approach was used in each CSM patient when performing the anterior surgery, while a standard midline posterior approach was used to the posterior cervical spine. Decompression was completed using a mixture of burrs and Kerrison rongeurs with removal of the posterior longitudinal ligament and visualization of the dura as the endpoint of anterior surgery. In posterior surgery, a laminectomy was performed after stabilization using lateral mass screws. The endpoint was the removal of the lamina and the ligamentum flavum.
We used either 5 (Group 1) or 9 (Group 2) stimuli. The MEPI criterion was a 100% increase in the MEP amplitude from baseline at the end of CSM surgery just prior to skin closure. We additionally examined MEPIs of 50%, 150%, and 200% in the intraoperative MEP amplitude from baseline. Since the data were collected prospectively, we first applied 5 pulses (Group 1) for 19 cases, followed by applying 9 pulses (Group 2), since the latter stimulus was noted to be more efficacious. Mann-Whitney U tests were used to compare between the two groups. Spearman's correlation test was used to test the relationships between two variables, with a p value of <0.05 considered to be statistically significant.
RESULTS
Forty-seven of the 69 patients were male. Age did not differ significantly between Group 1 (mean 62.4 years, range 39–85 years) and Group 2 (mean 65.3 years, range 31–82 years), nor did the surgical duration (mean 201.8 minutes vs. 197.3 minutes). The 19 patients in Group 1 comprised 12 with CSM, 3 with CSM, and 4 with ossification of the posterior longitudinal ligament (OPLL); the corresponding numbers among the 50 patients in Group 2 were 42, 3, and 5. None of the patients experienced postoperative clinical deterioration of muscle power. There were no reported intraoperative or postoperative complications. No significant blood loss or hypotension was encountered intraoperatively in any of the patients. Cervical OPLL was encountered in 10–20% of the patients. All of the OPLL patients had symptomatic cervical myelopathy.
The myelopathy level did not differ significantly between Groups 1 and 2, at 2.42±1.60 and 2.43±1.10 (p=0.63), nor did the MRC scale motor power in the upper limbs (4.2±0.4 vs. 4.3±0.5, p=0.43) or the lower limbs (4.1±0.3 vs. 4.2±0.4, p=0.33). None of the patients in either group showed deterioration in motor power in the review performed on postoperative day 1. An MEPI of 100% from baseline was observed in 10 patients (53%) in Group 1 and 36 patients (72%) in Group 2.
Table 1 summarizes the four definitions and their respective proportions in the patients with MEPI. Comparisons of the baseline mean MEP amplitudes of the five muscles bilaterally between Groups 1 and 2 did not reveal any significant differences (716 µV vs. 611 µV, z=0.076,
p=0.98). A higher percentage increase in the MEP amplitude was negatively correlated with a reduced proportion of patients with MEPI in Group 1 (
rs=−1,
p<0.005) and Group 2 (
rs=−1,
p<0.005).
Table 2 compares the total MEPI bilaterally for all five muscles between Groups 1 and 2 for an MEPI of 100%. A significant difference between the two groups was demonstrated (Mann-Whitney U test: z=−3.74,
p=0.00018). Further comparison between the 19 cases of Group 1 and the first 19 cases of Group 2 also revealed a significant difference (
p=0.0018), as indicated in
Table 3.
We compared the supramaximal stimulation intensity between the two groups. The required mean stimulation was significantly higher in Group 1 than in Group 2 (353 V vs. 238 V, z=−5.18,
p<0.00001), as shown in
Fig. 1.
We also studied another three patients who underwent anterior cervical discectomy and reconstruction with an allograft, cage, and screws. Two were performed at C5–C6 and one was performed at C3–C4, C4–C5, and C5–C6. Stimulation with five pulses resulted in MEPI being observed in four, two, and three muscle recordings; the corresponding response rates when stimulating at the same intensity with nine pulses were five, five, and seven muscle recordings (p=0.034). These findings further suggest that the nine-pulse stimulation protocol is more efficacious in activating descending motor neurons to elicit MEPI postoperatively.
Fig. 2 shows the actual IOM recordings of MEPI in one patient.
DISCUSSION
The findings of this study indicate that using nine-pulse stimulation (Group 2) required a lower intensity and resulted in a higher MEPI during CSM surgery than when using five-pulse stimulation (Group 1). This difference was evident despite the similarity of the baseline MEP amplitudes in the two groups, which were also comparable in terms of age, clinical diagnosis, and surgery duration.
MEPI was evident in more than 50% of the patients undergoing surgery, in contrast to previous studies
1112 finding much smaller proportions. However, direct comparisons with those studies might not be possible since the cortical stimulation parameters were not reported. It is interesting to note that the previous studies utilized a cutoff of 50% for MEPI, in contrast to the more-stringent criterion of 100% used in the present study, while the MEPI remained higher in the latter. While the stimulation intensities were also not described for the previous studies, we found that nine-pulse stimulation required lower intensities than five-pulse stimulation, and also resulted in a higher MEPI. Further examination of MEPI defined at from 50% to 200% of the baseline MEP amplitude (
Table 1) revealed a negative correlation with the proportion of patients showing MEPI, suggesting that an orderly linear relation exists between these two variables. This further suggests that future studies should redefine MEPI criteria in the continuing search for the most-representative one.
What is the physiological explanation for these findings? Multipulse cortical stimulation for IOM of spinal surgery aims to achieve an optimal summation of descending volleys at the spinal motor neuron in order to evoke a stable MEP of the highest amplitude.
1516 This process may be influenced by intraoperative factors, including anesthesia, as well as pathological situations of ischemia, blood loss, hypotension, vasospasm, and trauma. Utilizing a larger number of pulses at the same frequency may recruit additional descending motor tracts that excite spinal motor neuron pools. However, the baseline MEP amplitudes were comparable between Groups 1 and 2 in the present study, with the latter requiring a lower stimulation intensity. This suggests that prior to cervical cord decompression, similar numbers of descending volleys may be evoked by the two stimulation parameters. In contrast, the extra four pulses in Group 2 may evoke additional descending volleys after surgical decompression, which would facilitate this physiological process mechanically. The exact reasons are unclear, but it is possible that the initial five pulses had lowered the threshold of the cortical motor neurons to allow the additional four pulses to excite a larger pool of these neurons.
17 This phenomenon has been observed when using two pulse trains for cortical stimulation,
18 in which a conditioning train of three pulses followed by a test train of six pulses helps to increase the MEP amplitude. An extended train of pulses might also recruit indirect descending pathways mediated by interneurons. These mechanisms are also supported by a lower stimulation intensity being needed in Group 2.
Some controversy remains about the overall efficacy of surgery for CSM,
19 although recent data
20 have associated operative intervention with clinical benefit, most evidently at 2 years after the operation.
21 However, these findings are not based on IOM data. Two previous studies
1112 produced different results, correlating MEP improvement with better outcomes at 6 months and 1 month. However, the stimulation protocols were not stated explicitly and are actually unlikely to be comparable between the two studies, and their outcome measures were also dissimilar. Together with the controversy over the stimulus duration for achieving the maximum benefit in CSM surgery, we suggest that it is currently premature to draw conclusions about how MEPI influences the surgical outcome. Further studies utilizing larger samples, comparable and efficient stimulation protocols, and standardized outcome measures over longer follow-up periods may provide some of the answers.
It is also known that the intraoperative MEPI alone might not be adequate for predicting postoperative results. Several studies,
22 including ours,
5 have shown that cortical reorganization over 6 months and beyond may contribute to functional improvement during the postoperative recovery phase, possibly even up to 2 years later.
23 Improvement in upper limb dexterity has also been shown to be related to recruitment in motor areas including the postcentral gyrus, precentral gyrus, and premotor and supplementary motor areas
24 over a 6-month postoperative period and beyond. The single parameter of MEPI alone might not adequately assess the distributed plasticity changes other than reassuring the surgeon of the adequacy of decompression intraoperatively, provided that we know the optimal stimulation requirements explored here in the first instance. For these reasons, the current study was not designed to ascertain the prognosis of surgical decompression in relation to MEPI findings.
To our knowledge, this is the largest study of MEPI, and its results suggest that this dynamic phenomenon is observed in a much larger proportion of cases of cervical decompression surgery than previously reported. Intraoperative MEPI with long-train cortical stimulation may reflect the adequacy of decompression and provide additional guidance for the surgical procedure. Future studies correlating clinical improvements based on variable definitions of MEPI are warranted. MEPI can be defined as amplitude improvements ranging from 50% to 200%, and we remain uncertain about which definition is most strongly correlated with postsurgical outcomes. This is a limitation that should be addressed in future studies.
Figures and Tables
Fig. 1
Graphical illustration of the mean supramaximal stimulation intensities in Groups 1 and 2. Error bars indicate two standard deviations from the mean values.

Fig. 2
Actual IOM traces showing an MEPI of 100% from baseline in the left deltoid, right tibialis anterior, and right flexor carpi radialis recordings. The darker bottom traces in each muscle recording represent the baseline MEP, and the lighter trace is the MEP at the end of cervical decompression. The vertical gain and horizontal sweeps are indicated. IOM: intraoperative monitoring, MEP: motor evoked potential, MEPI: MEP improvement.

Table 1
Summary of 4 definitions and respective proportions of patients with MEPI

Table 2
Comparison of MEPI between Group 1 and 2 for individual muscles
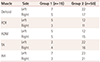
Table 3
Comparison of MEPI between Group 1 and 2 for individual muscles






 PDF
PDF ePub
ePub Citation
Citation Print
Print






 XML Download
XML Download