CASE REPORT
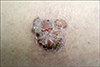
A 66-year-old male presented with a pruritic, 3×2 cmsized, erythematous brownish solitary plaque with crusted and verrucous surface on the extensor surface of the right thigh (
Fig. 1). The lesion developed 6 to 7 years ago, and had been growing slowly. There was no palpable inguinal lymph node enlargement, and the patient had been otherwise healthy. Biopsy revealed epithelial strands extending down to the dermis, forming anastomoses (
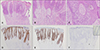
Fig. 2A). Epithelial strands were composed of 2 areas: the upper area consisting of dysplastic cells with prominent nucleoli and abundant mitoses, and the lower area consisting of oval and round cells, and occasionally small luminal ducts (
Fig. 2A~C). Dysplastic cells comprised almost the entire epidermis but did not invade into the dermis (
Fig. 2A). There was a sharp contrast and demarcation between the upper dysplastic lesion and the benign adenomatous lesion immediately surrounding the dysplastic keratinocytes in the lowermost portion of the epidermis (
Fig. 2A). In immunohistochemical stains, epithelial membrane antigen (EMA) was positive in the dysplastic cells and weakly positive in the ductal structures in the adenomatous lower portion (
Fig. 2D). p63 was more intensely positive in the dysplastic cells than in the adenomatous cells (
Fig. 2E). Carcinoembryonic antigen (CEA) stain was weakly positive in the ductal structures in the adenomatous lower portion (
Fig. 2F). The lesion was diagnosed as a reactive ESFA associated with Bowen's disease and was completely excised with free margins of the tumor. Histopathology of excised tissues was also consistent with reactive ESFA associated with Bowen's disease (
Fig. 3).
DISCUSSION
Although ESFA most often presents as a slowly growing, solitary, flesh- or reddish-colored nodule or plaque on the extremities of elderly patients, it has various clinical appearance ranging from a solitary erythematous scaly patch to multiple verrucous papules, nodules, and plaques
12. The distribution other than extremities includes face, trunk and rarely nails. ESFA is classified into 5 subtypes according to the clinical presentation: (1) solitary ESFA; (2) multiple ESFA with ectodermal dysplasia; (3) multiple ESFA without cutaneous findings; (4) unilateral linear ESFA; and (5) reactive ESFA. However, its histopathologic findings are peculiar and common to all subtypes. Typically, anastomosing strands and cords of monomorphous epithelial cells proliferate and are surrounded by abundant fibrous stroma. Occasionally small luminal eccrine ducts can be found within these cords
24.
Reactive ESFA, first described in 1997 by French
5 is a rare ESFA subtype associated with various inflammatory dermatoses or neoplasms. Venous stasis and ulceration is the most common dermatoses reported to be associated with ESFA
6, and others include trauma, hyperkeratotic eczema, burn scars, lichen planus, elephantiasis, bullous pemphigoid, eccrine poroma, and hidroacanthoma simplex
46. Even among the rare cases of reactive ESFA, ESFA associated with malignant neoplasm is extremely rare and there have been only 18 cases including the present case in the literature to date (
Table 1)
34678910111213141516. Of the 18 cases, SCC was the most commonly associated carcinoma, which was followed by SCC with porocarcinoma, porocarcinoma, BCC and Bowen's disease (SCC
in situ). Metastasis or recurrence after excision has not been reported. All but 2 cases occurred at the age older than 65 years; exceptional 1 case (case 1) occurred in a male's heel with repeated minor trauma, and the other 1 case (case 12) occurred in a female who had a history of numerous actinic keratoses. Dorsum of feet or hands (including fingers) was the most common site, and all 3 cases which occurred on the soles involved the heel area. Extremities were also involved with its extensor surface. The lesion duration showed a wide distribution ranging from 6 weeks to several decades. In most cases, lesions had been growing slowly and recently became painful or bled. Clinical appearance varied considerably ranging from palmoplantar keratoderma (case 2) to an exophytic mass approximately 10 cm in diameter (case 3). Histopathologic features were also variable. Five cases showed the histology of carcinoma with some benign ESFA foci, and conversely, 3 cases showed the histology of benign ESFA with some carcinomatous foci. In the other 10 cases, carcinomatous area and benign ESFA area were abutting contiguously; 1 case (case 3) showed SCC surrounded by ESFA, another 1 case (case 14) showed SCC alternating with ESFA, and other 2 cases (cases 8 and 9) showed mixed features of ESFA, SCC, and porocarcinoma. While there has been an attempt to distinguish carcinomatous area from benign ESFA with immunohistochemical stains, the results were inconsistent. CEA stains ductal structures regardless of whether it is benign or carcinomatous. Notwithstanding EMA, MNF116, p16, and p63 tend to stain carcinomatous area more strongly; however, they can stain benign ESFA area as well. It is still controversial whether ESFA precedes carcinoma and undergoes malignant transformation or if it is a reactive change secondary to carcinoma
3. Whereas SCC occurs most frequently on the head and neck and the upper extremity, most of cases occurred on the lower extremity, especially dorsum of the foot, extensor of the leg, or the heel, where the sites were prone to trauma. These findings implicate that venous insufficiency
7 or repeated minor trauma
2 might be associated with ESFA. No cases of reactive ESFA associated with carcinoma occurred in the head and neck region, the most common site of SCC. Overall, it can be postulated that SCC emerges from ESFA or occurs together with ESFA due to repeated minor trauma. Cases with duration of several decades also support the claim that ESFA precedes carcinoma. Some authors even proposed the term of ‘syringofibrocarcinoma’ to describe carcinoma associated with ESFA in this regard
8. In contrast, the hypothesis that ESFA is a secondary change to SCC seems reasonable with respect to cases of ESFA associated with numerous inflammatory or benign neoplastic dermatoses other than carcinoma.
Histopathologically, ESFA is similar to fibroepithelioma of Pinkus, poroma or porocarcinoma, hidroacanthoma simplex or malignant hidracanthoma simplex, and SCC with ductal differentiation. However, in fibroepithelioma of Pinkus, epithelial strands are composed of basaloid cells with stromal retraction, ductal structures within the strands are rare, and scattered mast cells are usually observed
9. Poroma shows epithelial strands which are similar to ESFA, but they rarely form anastomoses, and cystic and ductal structures are usually distinctly stained with EMA and CEA
917. Porocarcinoma shows marked cytologic atypia and numerous mitotic figures
9. An intraepidermal form of poroma and porocarcinoma is called hidroacanthoma simplex and malignant hidroacanthoma simplex, respectively. Although glandular proliferation is rarely observed in SCC, glandular components are typically negative for CEA, and prominent keratinization, keratin pearl and cytologic atypia are typically present in SCC in contrast to ESFA
18.
The treatment of choice is complete surgical excision since it is impossible to exclude malignant transformation of ESFA
12. When complete excision is difficult due to large involvement area or poor patient condition for surgery, close observation may be an alternative to early excision
2. An ESFA lesion that increases in size faster than the initial tumor, becomes painful or bleeds, or forms ulcer or crust is more likely to be associated with malignancy.
This report describes a rare case of ESFA associated with Bowen's diseases with literature review. ESFA associated with carcinoma usually presents with a solitary nodule on the lower extremity of the elderly, usually older than 60 years of age. Although it is uncertain whether ESFA precedes carcinoma or emerges from carcinoma, complete excision should be preferred and potential adjacent malignancy should be meticulously checked for when ESFA is diagnosed.






 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download