INTRODUCTION
Necrobiotic xanthogranuloma (NXG), is a rare multisystem disease, that manifests as generalized xanthomatous inflammatory skin lesions, and may be associated with paraproteinemia and other lymphoproliferative and hematologic disorders. It typically presents as indurated yellowred nodules or plaques, which are often ulcerated, frequently affecting the periorbital regions
1.
Diffuse normolipemic plane xanthoma (NX), is a rare acquired disease, first recognized by Altman and Winkelmann
2 in 1962. It is characterized by the presence of symmetric, yellowish flat or slightly elevated plaques distributed in the skin, and is often associated with systemic diseases, particularly multiple myeloma and monoclonal gammopathy
3.
Although immune complexes are thought to influence the pathophysiology of the above-mentioned diseases, the coexistence of these diseases has rarely been reported
4. We received the patient's consent form about publishing all photographic materials.
CASE REPORT
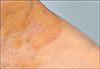
A 78-year-old female presented with yellowish patches on her neck, upper chest, and axillary areas. She was diagnosed with NX through a biopsy that showed numerous foamy cells in the dermis (
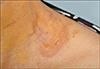
Fig. 1). She was advised to visit the hemato-oncologic department and undergo further evaluation, but she failed to do so. Four years later, she presented with an asymptomatic erythematous plaque in the middle portion of the NX lesion on her left supraclavicular area for 6 months. Dermatological examination revealed a child's fist-sized annular, erythematous plaque on a yellowish patch background (
Fig. 2). There was no remarkable past medical history and family history. Histopathological examination revealed lymphoid aggregates, collagen degeneration, granulomatous inflammation in the dermis. Moreover, numerous foreign-body, Touton type giant cells, foamy cells, and plasma cells were noticed in the higher magnification view (
Fig. 3). Cholesterol clefts, usually visualized within the necrobiotic foci of NXG, were not found. On laboratory testing, the hemoglobin level was 10.7 g/dl. The leukocyte count was 3.86 K/µl, with 49.8% polymorphonuclear leukocytes and 37.2% lymphocytes. There were 190,000 platelets/mm
3. The erythrocyte sedimentation rate was 120 mm/h. The total serum protein level was at 8.02 g/dl, with albumin level of 3.17 g/dl. The total cholesterol levels were at 191 mg/dl (the reference range is 120~200). Triglyceride and high-density lipoprotein (HDL)-cholesterol levels were 77 mg/dl and 58.1 mg/dl, respectively (the reference range is 35~160 and 45~65, respectively). Serum protein immunoelectrophoresis was done for possible paraproteinemia and showed an IgG kappa type monoclonal gammopathy. Bone marrow examination showed 20%~50% of cellularity with 15.6% plasma cells. The patient was diagnosed with multiple myeloma. Treatment was started with melphalan (Alkeran), bortezomib (Velcade) for multiple myeloma; and high-dose systemic corticosteroid, intralesional triamcinolone injection for NXG lesion. After 3 months of treatment, the erythematous skin lesion of NXG slightly improved, but the yellowish skin lesion of NX did not (
Fig. 4).
DISCUSSION
NXG and NX are two of the three distinct forms of xanthoma, and the other form is the hyperlipemic xanthoma (HX)
4. Xanthomas are characterized by the deposition of lipids (mainly cholesterol) in large foam cells, primarily in the skin and tendons. Although usually observed in the patients displaying dyslipidemia, xanthomatosis may also occur in cases of monoclonal gammopathy
5. HX and NX are characterized by yellow macules or papules, which make them look similar, but NX lesions are usually diffuse and plane, and HX lesions are more polymorphic, and can include tuberous, tendinous, palmar, or eruptive xanthoma
6. In contrast, NXG lesions are firm papules, nodules, or plaques, the color of which varies from yellow or red-orange to violaceous
7.
Histopathologic features of NXG are distinct. The epidermis may be normal or atrophic. NXG is always marked by granulomatous inflammation in the dermis, that can extend into the subcutaneous tissue. The granulomas consist of foamy histiocytes, lymphocytes, foreign body-type multinucleated giant cells, and Touton giant cells alternating with foci of collagen degeneration. Cholesterol clefts and nodular lymphoid aggregates are often reported. Special stains are not helpful in the diagnosis of NXG
8. The existence of foamy histiocytes, lymphocytes, foreign body-type multinucleated giant cells, and Touton giant cells in our case was consistent with the histologic findings of NXG. But cholesterol clefts were not found in our case.
Monoclonal gammopathy, associated with autoantibody activity against plasma lipoproteins, has been documented in NX and HX
68. Thus, work-up for possible paraprotemia is necessary. The autoantibody activity probably results in the formation of immune complexes and accumulation of cholesterol derivatives in the circulating monocytes and macrophages
9. The pathophysiology of NXG is currently not well understood, although cholesterol accumulation in the monocytes and its role in monoclonal gammopathy (MG) have been suggested
1011. Since MG has been considered to play a role in the pathophysiology of NX, HX, and NXG, the probability of coexistence of NXG in patients with NX was presumed to be higher than that in the general population. However, cases that reported the coexistence of NXG and NX at the same time were rare. NXG is clinicopathologically different from NX and HX in many respects; thus, its association with MG cannot be considered for these differences. Recently, Szalat et al.
12 suggested mechanism for the appearance of skin lesion in patients with NX and NXG. Low levels of anti-inflammatory HDL-C (apoA-I) were associated with increased expression of C-C chemokine receptor type 2 in circulating monocytes and elevated levels of chemokines (monocyte chemoattractant protein-1, interleukin-8, macrophage inflammatory protein-1α) and adhesion molecules (vascular cell adhesion molecule 1, intercellular adhesion molecule 1), which might favor monocyte recruitment and homing to skin in patients with NX and NXG. In NXG lesion, intermediate cluster of differentiation (CD) 14++CD16+ monocytes which were elevated in NXG, but not in NX and reported to be deleterious in pathologic conditions exacerbate this effect. This maybe the differentiating factor in clinicopathologically distinguishing NXG, from NX and HX. Although fluorescence-assisted cell sorting assay was not performed in our case, we presumed that CD14++CD16+ lymphocytes were increased due to an unknown mechanism, and the increase of these lymphocytes might cause inflammation of the existing NX lesions, resulting in NXG. Further studies on the association of CD14++CD16+ lymphocytes with MG are needed.
Various treatments have been reported for NXG with MG, but no treatment modality has been shown to be consistently effective. Treatment modalities include systemic or intralesional corticosteroids, chlorambucil, melphalan, topical mechlorethamine, dapsone, intravenous immunoglobulins, and lenalidomide
13. Bortezomib is a proteasome inhibitor, and is considered as a major milestone in the treatment of multiple myeloma. In 2008, the US FDA approved bortezomib as a first-line therapy of multiple myeloma
14. The first treatment case with bortezomib, for NXG-associated MG was reported in 2016, in which the paraproteinemia was normalized on treatment with bortezomib combined with low-dose systemic corticosteroid treatment, but the NXG skin lesion improved after high-dose dexamethasone therapy
14. This may underline that NXG is a reactive rather than a neoplastic skin disease. Our case is the second in the treatment trials of NXG and multiple myeloma with bortezomib, to the best of our knowledge. As in the previous case, bortezomib successfully improved MG in our case, and the high-dose corticosteroid and intralesional triamcinolone injection for the skin lesion of NXG were also effective.
In our case, NXG in the same region was more responsive to steroid treatment than NX, which was thought to be due to the suppression of intermediate CD14++CD16+ monocytes that cause inflammatory lesions. On the other hand, the treatment was less effective in NX, which was probably because the steroids did not significantly affect the removal of cholesterol-containing monocyte.
In conclusion, we recommend hematologic evaluation or long-term follow-up in patients with either NX, NXG, or HX because these diseases are associated with hematologic diseases. But, as it is difficult for clinicians to assess hematologic disease in all patients with these diseases, if a new NXG lesion develops in a patient with NX or HX, an evaluation of hematologic disease is strongly recommended because it can be an indication of any inflammatory response in the body.






 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download