INTRODUCTION
Atopic dermatitis (AD) and psoriasis have been traditionally considered as two different diseases, but recently, there has also been a view that both should be considered as a part of the same disease spectrum. The first evidence that they are different diseases is that they can be easily distinguished clinically
1. Another reason is the difference in the underlying genetic mechanism
2. However, there is also a view that considers both diseases as a part of the same spectrum because chronic AD shows features common with psoriasis on histology, such as epidermal hyperplasia, multiple T cells, and infiltration of dendritic cells (DCs)
13.
In terms of immunopathogenesis, AD is characterized primarily by the activity of T helper (TH)2 and TH22, while psoriasis is characterized primarily by the activity of TH1 and TH17
3. However, TH1 and TH17 are also involved in AD
3. In addition, a recent report showed that Asian AD patients show clinical patterns combined with psoriasis and TH17 polarization compared to Caucasian AD patients
4. This suggests that the immunophenotypes attributable to racial differences in AD might be different and that Asian AD might show an endophenotype similar to psoriasis
45. We assumed that there would be some patients with AD who showed psoriasiform features very similar to those of psoriasis histologically if Asian patients with AD were more affected by TH17 cytokine network. The purpose of this study was to investigate the clinical features, laboratory findings, and underlying immunophenotypes in Korean patients with chronic AD who showed psoriasiform features on histology.
MATERIALS AND METHODS
Tissue samples
Skin biopsy specimens were obtained from 59 adult Korean patients (aged >18 years) with AD and 15 adult patients with psoriasis for comparison from January 2009 to July 2016. Patients with AD were diagnosed based on the original Hanifin and Rajka diagnostic criteria
6. Patients with psoriasis were diagnosed clinically and with histopathology. The mean age of the AD patients was 19.9±3.2 years and that of the psoriasis patients was 27.0±6.4 (mean±standard deviation [SD]). This study was approved by the Institutional Review Board of Kyungpook National University Hospital (IRB no. KNUH 2016-12-001).
Histopathological evaluation and sub classification of AD patients
Skin samples were obtained from 59 Korean adult AD patients. We examined hematoxylin and eosin-stained sections of the skin biopsies. The slides were independently examined by three blinded observers for the following histologic parameters
7: acanthosis with regular elongation, thinning of the supra-papillary epidermis, pallor of the upper layers of epidermis, diminished to absent granular layer, confluent parakeratosis, the presence of Munro's microabscesses, elongation & edema of the dermal papillae, dilated capillaries, neutrophils in the epidermis, and eosinophils in the dermis. AD patients were segregated into one of the following groups based on the histopathological findings. To begin with, two dermato-pathologists and one dermatologist classified the psoriasiform chronic dermatitis (PCD) group (n=16) according to the typical histological features of psoriasis by consensus
7. Next, the patients were categorized into non-psoriasiform dermatitis with moderate-to-severe epidermal hyperplasia group (n=20) if there was moderate-to-severe epidermal hyperplasia with a presence of ≥7 layers, compact orthokeratosis, increased granular layer, broad-based acanthosis and vertically-oriented collagen bundles histologically. Finally, if there was only mild epidermal hyperplasia comprising 4~6 layers, we classified them as non-psoriasiform dermatitis with the mild epidermal hyperplasia group (n=23).
Clinical and Laboratory findings
Cutaneous manifestations were analyzed using clinical photographs of the patients and differences in the subclassification groups were compared. Patients with specific features, such as contact dermatitis, ichthyoses, seborrheic dermatitis, and other papulo-squamous diseases, were excluded from this study. Serum total immunoglobulin E (IgE), eosinophil count, and eosinophil ratio were measured in 47 patients, 56 patients, and 45 patients respectively. We compared the differences between the group showing PCD and the rest of the groups.
Stain and immunohistochemistry
Paraffin-embedded tissue sections of 3-µm thickness were processed for light microscopy. Immunohistochemistry for key cytokines in the immunopathogenesis of psoriasis, such as interleukin (IL)-12, IL-23, IL-17, IL-22, and interferon (IFN)-γ were performed using standard techniques. The primary antibodies included: IL-23 (diluted to a concentration of 2.5 µg/ml; ab189300; Abcam, Cambridge, MA, USA), IL-17 (diluted 1:50; sc-7927; Santa Cruz Biotech, Santa Cruz, CA, USA), IL-22 (diluted 1:500; ab18499; Abcam), IL-12 (diluted to a concentration of 8 µg/ml; MAB611; R&D systems, Minneapolis, MN, USA), and IFN-γ (diluted to a concentration of 1 µg/ml; ab25101; Abcam).
Image analysis
For the quantification of IL-23, IL-17, IL-22, and IFN-γ in the epidermis, the stained area (SA) per epidermal area (EA) was measured in the lesion. Each measurement was evaluated under a constant magnification. For each frame, the tracing was repeated 3 times and the mean was determined. The image was analyzed using software (Image-Pro Plus, version 4.5; Media Cybernetics Inc., Bethesda, MD, USA). All morphometric procedures were performed by manually tracing the borders of the epidermis and rete ridges. The EAs containing hair follicles were excluded from this tracing.
Semi-quantification of the IL-12 expression in the dermis was graded on a scale of 0 to 4: 0, no staining; 1, weak focal staining; 2, moderate staining; 3, diffuse moderate to pronounced staining; 4, very pronounced staining. All slides were evaluated by two independent dermatologists in a blinded fashion.
Statistics
Statistical analysis was performed using a software (SPSS ver. 11.0; SPSS Inc., Chicago, IL, USA). The Mann–Whitney U-test was used to determine the statistical significance in the differences between the groups. A value of p of <0.05 was considered statistically significant. Data were expressed as the mean±SD.
RESULTS
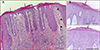
Fifteen of 59 AD patients (25.4%) showed psoriasiform appearance on histopathology (
Fig. 1A,
Table 1). Histopathological findings similar to those of Munro's microabscess were observed in 4 of 15 cases with psoriasiform features (
Fig. 1B). As mentioned above, we classified the tissues of patients with AD into 3 groups: PCD group, group showing non-psoriasiform dermatitis with moderate to severe epidermal hyperplasia, and group with non-psoriasiform dermatitis with mild epidermal hyperplasia based on acanthosis as the key histological feature. Clinical features of the patients with psoriasiform type often showed clearer lesion boundaries and noticeable scaling. Laboratory findings showed that there was no significant difference in the total IgE level and eosinophil count between the groups (
Fig. 2).
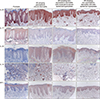
To determine whether histologic differences were based on the underlying immunophenotype, immunohistochemical staining was performed for IL-12 and IL-23, the key regulatory cytokines, and IL-22, IL-17, and IFN-γ, the effector cytokines in psoriasis, for comparison and analysis of the extent of expression in tissues. The expression pattern was first checked in the psoriatic tissues. The stained inflammatory cells were found to be mostly DCs. IL-23, a regulatory cytokine, was found to have a strong stain in the epidermis and dermal inflammatory cells. IL-17, a typical effector cytokine, was also stained throughout the epidermis. IL-22 was weakly expressed in all layers of the epidermis except for the basal layer. IL-12, another regulatory cytokine, was barely stained in the epidermis and strongly stained in the dermal inflammatory cells. IFN-γ was stained usually in the upper part of the epidermis (
Fig. 3).
In all 3 AD groups, IL-23 was strongly expressed in the basal layer within rete ridge. In both epidermis and dermis, IL-23 expression did not differ between the four groups (
Fig. 3,
Table 2). IL-17, affected by IL-23, was weakly stained in the PCD group, compared to the psoriasis group. Weak staining in the other 2 AD subgroups was also found seen on image analysis (
Fig. 3,
Table 2). IL-22, another cytokine affected by IL-23, had a stronger stain than the psoriatic lesions in the PCD group. This pattern was found in the other 2 AD subgroups (
Fig. 3,
Table 2) as well.
In the PCD group, IL-12 staining was similar to that of the psoriasis lesions in dermal inflammatory cells with dendrites that is supposed to be dermal DCs. Unlike IL-23, IL-12 was strongly expressed in dermal inflammatory cells in the PCD group compared to the 2 other AD subgroups (
Fig. 3,
Table 2). For IFN-γ, the PCD group showed a homogeneous staining pattern in the entire epidermis unlike in psoriasis, wherein only the upper part of the epidermis was stained. The staining intensity in the PCD group was not significantly different from that of psoriasis group. IFN-γ showed a similar degree of staining in all the 3 AD groups (
Fig. 3,
Table 2).
DISCUSSION
AD and psoriasis are the most common inflammatory skin diseases and are clinically different. AD lesions are characterized by blunted red lichenified plaques, mainly on the flexor surfaces, with nonadherent white scales. In contrast, psoriasis lesions are characterized by well-demarcated, dry, bright-red plaques with thick, nonadherent, silvery-white scales on the extensor surfaces
1. However, chronic AD has many histologic similarities with psoriasis, including increased epidermal hyperplasia and infiltrates of large numbers of T cells and DCs. At the same time, recruitment of T cells into the skin and their effector responses are considered to be key features in the pathogenesis of AD and psoriasis
891011. In both diseases, expansion of the cutaneous lymphocyte-associated antigen-positive T cell subsets T cell subsets are present in the skin lesions
1213. However, the subset of immune cells that localize at the site of the inflamed skin are different. AD is mediated by TH2 and TH22 cells; TH1 and TH17 cells also contribute to the AD immunopathogenesis. In contrast, psoriasis is mainly caused by TH1 and TH17 cells.
Recent studies have shown that immune characteristics in Asian patients are distinct from those in the European American, specifically for immune modulating molecules such as high expression of TH17 and TH2 elements, reinforcing the clinical importance of ethinicity in patients
45. They observed that the skin lesions of Asian AD patients showed a mixed phenotype of psoriasis and AD, compared to the European American patients, which led us to investigate the comprehensive immunological characteristics in Asian patients with AD
4. In this study, we observed 15 AD cases showing psoriasiform features on histology. There was no significant difference in the IgE levels and eosinophil count among the three AD subgroups, classified by their histologic differences. However, there are the distinct clinical differences among them, which inspired us to investigate the immunological relevance between the clinical manifestations of Asian AD and the pathological mechanism of psoriasis at the molecular cytokine level.
Although factors triggering psoriasis are not well defined, it is known that the innate immune system responds by releasing cytokines that activate DCs. Once activated, the DCs produce the regulatory cytokines IL-12 and IL-23, which is a key step in the development of psoriasis. IL-12 drives the differentiation of type-1 helper T-cells, or TH1 cells, which then produce more of the effector cytokines IFN-γ and tumor necrosis factor-α. In turn, IL-23 helps to promote the survival and proliferation of TH17 and TH22 cells, which secrete the effector cytokines IL-17A, F and IL-22, respectively. These effector cytokines directly cause the keratin site activation and proliferation, epidermal hyperplasia, and chronic inflammation that characterizes psoriasis. Due to their roles in psoriasis, both effector and regulatory cytokines are the targets of biologic therapies in use or under investigation.
In this study, we examined the effect of these cytokines on the histology of PCD. According to the immunohistochemical staining results, immuno-phenotypes in patients with chronic AD with psoriasiform appearance showed an increase in IL-23, IL-22, and IL-12 without an increase in IL-17. Expression of IL-23 was increased in three AD subgroups, but the expression of IL-12 was increased only in the PCD group. IL-17 expression in PCD the group was not extensive, contrary to what was expected. We presume that the weak expression of IL-17 despite a strong expression of IL-23 was due to the TH2 cytokine environment of AD. This indicates that TH2 cytokines, such as IL-4 and IL-13, might decrease the TH17 cell activity
1. IFN-γ was not expressed more strongly in all the AD groups compared to the psoriasis group.
Some cases of treatment with ustekinumab, an IL-12 and IL-23 inhibitor, in patients with chronic AD have been reported, but its efficacy remains controversial. Recent studies have shown that the effect of ustekinumab seems to be minimal
1415. However, we believe that the patient categorization of these studies should be re-assessed based on their histological manifestations because our current study found that 25.4% of patients had PCD. This can underestimate the efficacy of ustekinumab in specialized patient groups without specific selection of the immunophenotype for AD patients. Therefore, further studies on the effect of ustekinumab in AD patients with PCD are necessary. The main limitation of this study is that the expression of several factors associated with psoriasis was investigated using only immunohistochemical methods. Therefore, further research at the molecular level is needed to elucidate the relationship between the various cytokines from inflammatory cells and the formation of psoriasiform features in chronic AD.
AD can exhibit diverse and changing clinical patterns due to the more complex etiological factors compared to those of psoriasis. Patient selection might be more important than adjustment of the drug dose or interval to increase the efficacy of biologics used in the treatment of psoriasis in patients with AD. This study is clinically significant in that provide basic data for customized biologics therapy in patients with severe AD.






 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download