1. Muddugangadhar BC, Amarnath GS, Sonika R, Chheda PS, Garg A. Meta-analysis of failure and survival rate of implant-supported single crowns, fixed partial denture, and implant tooth-supported prostheses. J Int Oral Health. 2015; 7:11–17.
2. Simonis P, Dufour T, Tenenbaum H. Long-term implant survival and success: a 10-16-year follow-up of non-submerged dental implants. Clin Oral Implants Res. 2010; 21:772–777.
3. Staubli N, Walter C, Schmidt JC, Weiger R, Zitzmann NU. Excess cement and the risk of peri-implant disease - a systematic review. Clin Oral Implants Res. 2017; 28:1278–1290.
4. Wittneben JG, Millen C, Brägger U. Clinical performance of screw- versus cement-retained fixed implant-supported reconstructions-a systematic review. Int J Oral Maxillofac Implants. 2014; 29:84–98.
5. Wittneben JG, Joda T, Weber HP, Brägger U. Screw retained vs. cement retained implant-supported fixed dental prosthesis. Periodontol 2000. 2017; 73:141–151.
6. Amorfini L, Storelli S, Mosca D, Scanferla M, Romeo E. Comparison of cemented vs screw-retained, customized computer-aided design/computer-assisted manufacture zirconia abutments for esthetically located single-tooth implants: A 10-year randomized prospective study. Int J Prosthodont. 2018; 31:359–366.
7. Lemos CA, de Souza Batista VE, Almeida DA, Santiago Júnior JF, Verri FR, Pellizzer EP. Evaluation of cement-retained versus screw-retained implant-supported restorations for marginal bone loss: A systematic review and meta-analysis. J Prosthet Dent. 2016; 115:419–427.
8. Lee JI, Lee Y, Kim NY, Kim YL, Cho HW. A photoelastic stress analysis of screw- and cement-retained implant prostheses with marginal gaps. Clin Implant Dent Relat Res. 2013; 15:735–749.
9. Cicciu M, Bramanti E, Matacena G, Guglielmino E, Risitano G. FEM evaluation of cemented-retained versus screw-retained dental implant single-tooth crown prosthesis. Int J Clin Exp Med. 2014; 7:817–825.
10. Tanimura R, Suzuki S. Comparison of access-hole filling materials for screw retained implant prostheses: 12-month in vivo study. Int J Implant Dent. 2017; 3:19.
11. Hebel KS, Gajjar RC. Cement-retained versus screw-retained implant restorations: achieving optimal occlusion and esthetics in implant dentistry. J Prosthet Dent. 1997; 77:28–35.
12. Taylor RC, Ghoneim AS, McGlumphy EA. An esthetic technique to fill screw-retained fixed prostheses. J Oral Implantol. 2004; 30:384–385.
13. Weininger B, McGlumphy E, Beck M. Esthetic evaluation of materials used to fill access holes of screw-retained implant crowns. J Oral Implantol. 2008; 34:145–149.
14. Sancho-Puchades M, Crameri D, Özcan M, Sailer I, Jung RE, Hämmerle CHF, Thoma DS. The influence of the emergence profile on the amount of undetected cement excess after delivery of cement-retained implant reconstructions. Clin Oral Implants Res. 2017; 28:1515–1522.
15. Gehrke P, Bleuel K, Fischer C, Sader R. Influence of margin location and luting material on the amount of undetected cement excess on CAD/CAM implant abutments and cement-retained zirconia crowns: an in-vitro study. BMC Oral Health. 2019; 19:111.
16. Ma S, Fenton A. Screw- versus cement-retained implant prostheses: a systematic review of prosthodontic maintenance and complications. Int J Prosthodont. 2015; 28:127–145.
17. Papakostas GI, McGrath P, Stewart J, Charles D, Chen Y, Mischoulon D, Dording C, Fava M. Psychic and somatic anxiety symptoms as predictors of response to fluoxetine in major depressive disorder. Psychiatry Res. 2008; 161:116–120.
18. Vaillancourt H, Pilliar RM, McCammond D. Finite element analysis of crestal bone loss around porous-coated dental implants. J Appl Biomater. 1995; 6:267–282.
19. Bulaqi HA, Mousavi Mashhadi M, Safari H, Samandari MM, Geramipanah F. Effect of increased crown height on stress distribution in short dental implant components and their surrounding bone: A finite element analysis. J Prosthet Dent. 2015; 113:548–557.
20. Niinomi M. Mechanical properties of biomedical titanium alloys. Mater Sci Eng. 1998; 243:231–236.
21. Sertgöz A. Finite element analysis study of the effect of superstructure material on stress distribution in an implant-supported fixed prosthesis. Int J Prosthodont. 1997; 10:19–27.
22. Tolidis K, Papadogiannis D, Papadogiannis Y, Gerasimou P. Dynamic and static mechanical analysis of resin luting cements. J Mech Behav Biomed Mater. 2012; 6:1–8.
23. Lang LA, Kang B, Wang RF, Lang BR. Finite element analysis to determine implant preload. J Prosthet Dent. 2003; 90:539–546.
24. Guda T, Ross TA, Lang LA, Millwater HR. Probabilistic analysis of preload in the abutment screw of a dental implant complex. J Prosthet Dent. 2008; 100:183–193.
25. Wang RF, Kang B, Lang LA, Razzoog ME. The dynamic natures of implant loading. J Prosthet Dent. 2009; 101:359–371.
26. Silva GC, Cornacchia TM, de Magalhães CS, Bueno AC, Moreira AN. Biomechanical evaluation of screw- and cement-retained implant-supported prostheses: a nonlinear finite element analysis. J Prosthet Dent. 2014; 112:1479–1488.
27. Lee H, Park S, Noh G. Biomechanical analysis of 4 types of short dental implants in a resorbed mandible. J Prosthet Dent. 2019; 121:659–670.
28. Roberts WE, Sarandeep H, Jeffery AR. Bone modeling: biomechanics, molecular mechanisms, and clinical perspectives. Semin Orthod. 2004; 10:123–161.
29. Torcato LB, Pellizzer EP, Verri FR, Falcón-Antenucci RM, Santiago Júnior JF, de Faria Almeida DA. Influence of parafunctional loading and prosthetic connection on stress distribution: a 3D finite element analysis. J Prosthet Dent. 2015; 114:644–651.
30. Santiago Junior JF, Verri FR, Almeida DA, de Souza Batista VE, Lemos CA, Pellizzer EP. Finite element analysis on influence of implant surface treatments, connection and bone types. Mater Sci Eng C Mater Biol Appl. 2016; 63:292–300.
31. Ramos Verri F, Santiago Junior JF, de Faria Almeida DA, de Oliveira GB, de Souza Batista VE, Marques Honório H, Noritomi PY, Pellizzer EP. Biomechanical influence of crown-to-implant ratio on stress distribution over internal hexagon short implant: 3-D finite element analysis with statistical test. J Biomech. 2015; 48:138–145.
32. Minatel L, Verri FR, Kudo GAH, de Faria Almeida DA, de Souza Batista VE, Lemos CAA, Pellizzer EP, Santiago JF Junior. Effect of different types of prosthetic platforms on stress-distribution in dental implant-supported prostheses. Mater Sci Eng C Mater Biol Appl. 2017; 71:35–42.
33. He Y, Fok A, Aparicio C, Teng W. Contact analysis of gap formation at dental implant-abutment interface under oblique loading: A numerical-experimental study. Clin Implant Dent Relat Res. 2019; 21:741–752.
34. Robinson D, Aguilar L, Gatti A, Abduo J, Lee PVS, Ackland D. Load response of the natural tooth and dental implant: A comparative biomechanics study. J Adv Prosthodont. 2019; 11:169–178.
35. Mahnama A, Tafazzoli-Shadpour M, Geramipanah F, Mehdi Dehghan M. Verification of the mechanostat theory in mandible remodeling after tooth extraction: animal study and numerical modeling. J Mech Behav Biomed Mater. 2013; 20:354–362.

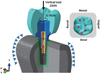

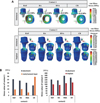
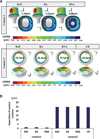
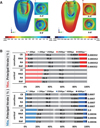




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download