Abstract
Objective
The objective of this study was to evaluate the effects of cetirizine, a histamine 1 receptor antagonist, on bone remodeling after calvarial suture expansion.
Methods
Sixty male Sprague–Dawley rats were divided into 4 groups; the phosphate-buffered saline (PBS)-injected no expansion group, cetirizine-injected no expansion group, PBS-injected expansion group, and cetirizine-injected expansion group, and were observed at 7, 14, and 28 days. Five rats per group were examined at each observation day. Daily injections of cetirizine or PBS were administered to the relevant groups starting 2 weeks prior to expander insertion. A rapid expander was inserted in the calvarial bone to deliver 100 cN of force to the parietal suture. The specimens were prepared for hematoxylin and eosin and tartrate-resistant acid phosphatase (TRAP) staining. Suture opening and bone regeneration were evaluated using microcomputed tomography and bone histomorphometric analysis. Serum blood levels of osteocalcin and carboxy-terminal collagen crosslinks (CTX) were also evaluated.
Results
TRAP-positive cell counts and CTX levels decreased while osteocalcin levels increased in the cetirizine-injected expansion group at observation day 28. In the expansion groups, the mineralized area gradually increased throughout the observation period. At day 28, the cetirizine-injected expansion group showed greater bone volume density, greater mineralized area, and narrower average suture width than did the PBS-injected expansion group.
Epidemiological studies have suggested a causative relationship between environmental pollution and the increased incidence of allergic conditions in the general population.1 Recently, several studies have shown an increase in atopic dermatitis and asthma in urban children, and this has been associated with air pollution and proximity to traffic.2345 Histamine is a major mediator in allergic diseases and atopic dermatitis, which are broadly managed by prescribing antihistamines for long durations.67
Histamine affects bone remodeling by modulating the osteoclastic pathway, which increases bone resorption.89 Animal studies have shown that blocking the uptake of histamine by using histamine receptor antagonists decreases osteoclastic activity as well as bone resorption. In rats, histamine 1 receptor antagonists (H1RAs) have been shown to increase the mechanical properties of the bone by inhibiting bone resorption.1011
Allergic rhinitis, which is mainly treated using antihistamines, commonly causes upper airway obstruction that is a significant risk factor for developing malocclusion in children.121314 The number of children with such allergic symptoms has increased continuously during the past years.15 Therefore, it is conceivable that children with different malocclusions may be taking antihistamines sometime during their orthodontic treatment. A previous study on rats also showed a decrease in alveolar bone resorption, which in turn decreased tooth movement upon antihistamine injection.16 Young children with malocclusions often need dentofacial orthopedic treatment involving growth modification via craniofacial suture expansion followed by new bone formation, which could be affected by antihistamine intake. Therefore, the aim of this study was to evaluate the effects of cetirizine, a H1RA, on bone remodeling after calvarial suture expansion in rats.
Sixty male Sprague–Dawley rats (weight, 280–315 g; age, 8 weeks old) were housed in standard breeding cages under normal temperatures (22–24℃) and 12-hour light/dark conditions, and were provided standard rat chow. The rats were randomly divided into four groups: phosphate-buffered saline (PBS)-injected no expansion, cetirizine-injected no expansion, PBS-injected expansion, and cetirizine-injected expansion (Figure 1). Five rats from each group were observed at 7, 14, and 28 days, and were weighed at the beginning and end of the observation periods (Figure 2). All procedures in this animal study complied with the Guideline of the Association for Assessment and Accreditation of Laboratory Animal Care International, and were approved by the Institutional Animal Research Ethics Committee at the Yonsei Medical Center (IACUC Approval number, 2017-0176).
Cetirizine (10 mg/kg; Sigma-Aldrich, St. Louis, MO, USA) or PBS (10 mg/kg) was injected peritoneally on a daily basis to the relevant groups 2 weeks prior to expansion device insertion. The rapid expander was constructed using a stainless-steel round wire having a diameter of 0.5 mm (remanium®; Dentaurum, Ispringen, Germany). The device was designed to meet the dimensions of 10-mm width and 10-mm height with 1 helical spring at the end when inactivated. To deliver the expansion force, the appliance was placed on a grid and the ends were expanded to deliver a force of 100 cN (Figure 1). General anesthesia was administered for 2 minutes by using 5% isoflurane (Ifran; Hana Pharm, Seoul, Korea) in oxygen (50 mg/kg), followed by an intraperitoneal injection of Zoletil 50 (Virbac, Carros, France) at 30 mg/kg and xylazine (Rompun®; Bayer Healthcare, Seoul, Korea) at 10 mg/kg of body weight. A high-speed 330 carbide bur of 0.8-mm diameter was used to drill a small hole on each side of the parietal bones in the calvarial bone with a 10-mm distance in between to fit the appliance in an inactivated state. Thereafter, the appliance was inserted either in an inactive or expanded state. The helical spring was fixed with resin after insertion. Daily injections of cetirizine (Sigma–Aldrich) at 10 mg/kg or PBS at 10 mg/kg were administered approximately at the same time throughout the 7-, 14-, and 28-day periods. All animals were sacrificed after the experimental period on days 7, 14, and 28.
The rat calvarial bone was dissected on days 7, 14, and 28 to include the bilateral parietal bones, sagittal suture, and coronal suture (Figure 1). All specimens were fixed with 4% paraformaldehyde solution for 2 weeks after dissection. For hematoxylin and eosin staining (Sigma–Aldrich), the fixed specimens were decalcified with 10% ethylenediaminetetraacetic acid (pH 7.4) at 4℃ for 3 weeks, embedded in paraffin, and sectioned at a thickness of 4 µm (Figure 3). For tartrate-resistant acid phosphatase (TRAP) staining (Sigma-Aldrich), the sections were dewaxed, rehydrated, and incubated for 1 hour at 37℃ in the TRAP reagent.
For each specimen, 3 areas with a total area of 9 × 104 µm2 were randomly selected with a region of interest at the center of the suture. Each area was analyzed using the MetaMorph software (Molecular Devices, Sunnyvale, CA, USA), and the results were averaged for obtaining the mineralized area (µm2), fibrous area (µm2), and mineralized/fibrous area ratio (Figure 4). The measurements were taken in reference to a previous study by Parfitt et al.17 TRAP-positive cell counts were performed on the 3 selected areas and were averaged for each group (Figure 5).
The samples were scanned using microcomputed tomography (micro-CT) (SkyScan 1173; Bruker, Kontich, Belgium) conducted at 130 kV and 60 µA with a pixel size of 30 µm. The region of interest was determined at 2 mm from the coronal suture with a 4- × 4-mm-sized square area and a depth of 0.7 mm, including the sagittal suture focused at the center (Figure 1).
The morphometric indices of the sagittal suture area were evaluated using the CTAn® software (SkyScan; Bruker) and the bone volume density (BV, mm3), bone/tissue volume percentage (BV/TV, %), and average suture width (mm) were assessed. The average suture width was calculated using the following equation:
Average suture width = [(total volume − bone volume) of the region of interest] / 4 / 0.7.
Blood samples were collected from the lateral tail vein at 2 time points: at the day of expansion appliance insertion and at the time of sacrifice on days 7, 14, and 28. Serum was separated by centrifugation and stored at −80℃. Serum protein levels of osteocalcin and carboxyterminal collagen crosslinks (CTX) were measured using the osteocalcin enzyme-linked immunosorbent assay (ELISA) kit (MyBioSource, San Diego, CA, USA) and CTX ELISA kit (MyBioSource). Optical density was measured at a wavelength of 450 nm.
Statistical analysis was performed using SAS software ver. 9.4 (SAS Institute, Cary, NC, USA). The normality of the variables was tested using the Kolmogorov–Smirnov test. Descriptive statistics were represented as mean and standard deviation. Difference between groups was analyzed using one-way analysis of variance followed by post-hoc least significant difference tests. Statistical significance was set at p < 0.05.
The initial age and weight of the rats did not differ significantly among the groups. Rats with active expansion devices did not have a significant difference in weight compared to those with inactive expansion devices in both the PBS- and cetirizine-injected groups during all the observation periods. However, rats injected with cetirizine weighed less at the 7- and 28-day observation periods (Figure 2).
In the expansion groups, the micro-CT images displayed suture closure via gradual bone formation during the 7-, 14-, and 28-day observation periods. At the 28-day observation period, the cetirizine-injected expansion group had significantly greater BV and BV/TV as well as narrower average suture width than did the PBS-injected expansion group. No significant differences were observed between the no expansion groups in BV, BV/TV, and average suture width during all the observation periods (Table 1).
On histological examination, the expansion groups showed expansion of the parietal suture with fibrous tissue reoriented across the suture parallel to the direction of expansion, with immature woven bone at the suture margins; however, the no expansion groups showed no disruption of the sutural fibers and showed traces of only small amounts of new bone (Figure 3). Histomorphometric analysis of the parietal suture area showed a significantly smaller mineralized area in the expansion groups than in the no expansion groups because of active expansion of the sutures. The mineralized area gradually increased in the expansion groups during the observation periods, and at the 28-day observation period, the cetirizine-injected expansion group had a significantly greater mineralized area and mineralized area/fibrous area ratio than did the PBS-injected expansion group (Table 2, 3). The number of TRAP-positive cells at the 28-day observation period was smaller in the cetirizine- injected expansion group than in the PBS-injected expansion group (Figure 5).
The cetirizine-injected groups had higher serum osteocalcin levels than did the PBS-injected groups at the 14- and 28-day observation periods. At day 28, serum CTX levels were significantly lower in the cetirizine-injected expansion group than in the PBS-injected expansion group (Figure 6).
Sutures play an important role as growth sites in the craniofacial region. Dentofacial orthopedic devices are designed to utilize the expansion potentials of the sutures to modify undesirable growth patterns and skeletal discrepancies. In such processes, mechanical stimuli are exerted on the sutures, and these activate the cellular growth responses in the sutures.18 Studies have shown that cartilaginous tissue proliferates and differentiates at the osteogenic layer in the bone fronts of the suture when mechanical expansion force is applied.1920 Several animal studies have shown that tensional forces progressively widen the sutures and that cartilaginous tissue is replaced by new bone formation in irregular patterns that parallel the stretching force.2122 In this study, the histological evaluations showed finger-like projections of new non-lamellar bone starting from the ends of the parietal bones and extending irregularly towards the center of the expanded sutures; this was similar to the findings of previous studies using expansion devices on rat sutures.2123
Bone remodeling occurs via sequential steps that are mediated by osteoclasts resorbing old bone and osteoblasts depositing new bone. Histamine has been reported to promote osteoclastogenesis by acting on the differential expression of histamine receptors on osteoblasts and osteoclasts.24 A study on ovariectomized rats showed impaired bone resorption after histamine receptor antagonist treatment because of a decrease in both osteoclast recruitment and resorption.25 Likewise, many studies have evaluated different histamine receptor antagonists and their effects on the balance of bone remodeling.102627 In this study, the results of micro-CT and histomorphometric analysis showed a significant increase in BV, BV/TV percentage, mineralized area, and mineralized/fibrous area ratio in the cetirizine-injected expansion group, which was in line with those of a previous study by Meh et al.16 that showed an increase in alveolar BV at a late phase during orthodontic tooth movement due to cetirizine injections.
Osteocalcin is a bone matrix protein synthesized by osteoblasts, and it is used as marker for bone formation.28 The blood serum osteocalcin levels were significantly higher in the cetirizine-injected groups at days 14 and 28, indicating active bone formation. During bone resorption, collagen fragments, such as serum CTX are generated, and CTX is used as a marker for osteoclastic activity.29 CTX level was significantly lower in the cetirizine-injected expansion group than in the PBS-injected expansion group at day 28. A decrease in the TRAP-positive cell count on day 28 in the cetirizine-injected expansion group was also noted, which was in line with the systematic effect of cetirizine observed via the blood serum CTX levels. An increase in osteocalcin and decrease in CTX levels as well as a decrease in the TRAP-positive cell count on day 28 in the cetirizine-injected expansion group also support the micro-CT findings showing higher bone volumes at day 28 after cetirizine injection.
This study calculated the average suture width by subtracting the bone volume from the total volume in the region of interest. This method was used because of the irregular shape of the cranial suture, which is a limitation of this study in terms of comparing the actual amount of suture expansion at a specific point. When using this method, the average suture width was not significantly different between the cetirizine- and PBS-injected expansion groups at day 7, suggesting that even though the animals were preinjected with cetirizine or PBS 2 weeks before expander insertion, an intake of antihistamines does not clearly affect the amount of rapid suture expansion. However, a significant increase was observed in bone formation at a microscopic level at day 28, which was most likely due to decreased bone resorption caused by a H1RA suppressing osteoclastic activity. The positive influence of cetirizine on new bone formation suggests that the use of a H1RA may be beneficial in terms of bone formation after mechanically stimulated cranial suture expansion. Nevertheless, further studies evaluating the influence of different categories and dosages of antihistamines on bone remodeling in the craniofacial suture region are needed for verifying the clinical significance of these findings.
This study observed the effects of cetirizine, a H1RA, on bone remodeling after suture expansion in rats. The main findings were as follows: 1) The amount of rapid suture expansion was not clearly affected by cetirizine administration. 2) Cetirizine increased bone formation in the expanded suture area at day 28 by suppressing osteoclast activity. 3) Intake of a H1RA may aid in bone formation after mechanical expansion of cranial sutures in the rat model.
Figures and Tables
Figure 1
Three-dimensional microcomputed tomography (micro-CT) reconstruction of the phosphate-buffered saline (PBS)/cetirizine groups after active expansion of the parietal suture at 7 (A, D), 14 (B, E), and 28 (C, F) days. Parietal suture showing the region of interest (ROI) used in the micro-CT analysis (G). Parietal suture with the expansion appliance (H). Images of the PBS- and cetirizine-injected no expansion groups (I, J).

Figure 2
Effect of cetirizine/phosphate-buffered saline (PBS) and active/inactive expansion on body weight.

Figure 3
Changes of the suture on histological sections. Hematoxylin and eosin staining of the parietal suture. Sevenday phosphate-buffered saline (PBS) no expansion (A), 7-day PBS expansion (A′), 14-day PBS no expansion (B), 14-day PBS expansion (B′), 28-day PBS no expansion (C), 28-day PBS expansion (C′), 7-day cetirizine no expansion (D), 7-day cetirizine expansion (D′), 14-day cetirizine no expansion (E), 14-day cetirizine expansion (E′), 28-day cetirizine no expansion (F), and 28-day cetirizine expansion (F′). Scale bar = 100 µm.
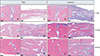
Figure 4
Region of interest used in the histomorphometric analysis. Representations of the mineralized area (A), voided areas (B), and fibrous area (C). H&E staining (left, ×20 magnification; right, ×40 magnification). Scale bar = 100 µm.
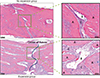
Figure 5
Tartrate-resistant acid phosphatase (TRAP)-positive cell count comparison between the phosphate-buffered saline (PBS)- and cetirizine-injected rats in the no expansion and expansion groups. TRAP staining. Scale bar = 20 µm.
***p < 0.001.

Figure 6
Comparison of osteocalcin and carboxy-terminal collagen crosslink (CTX) levels between the phosphate-buffered saline (PBS)- and cetirizine-injected rats in the no expansion (A, B) and expansion (C, D) groups.
*p < 0.05, **p < 0.01, ***p < 0.001.

Table 2
Comparison of the mineralized area, fibrous area, and mineralized/fibrous area ratio in the no expansion group

ACKNOWLEDGEMENTS
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIP; Ministry of Science, ICT & Future Planning) (NRF-2017R1D1A1B03030851).
Notes
References
2. Hajar T, Simpson EL. The rise in atopic dermatitis in young children: what is the explanation? JAMA Netw Open. 2018; 1:e184205.
3. Song S, Lee K, Lee YM, Lee JH, Lee SI, Yu SD, et al. Acute health effects of urban fine and ultrafine particles on children with atopic dermatitis. Environ Res. 2011; 111:394–399.


4. Xu F, Yan S, Li F, Cai M, Chai W, Wu M, et al. Prevalence of childhood atopic dermatitis: an urban and rural community-based study in Shanghai, China. PLoS One. 2012; 7:e36174.

5. Kim YM, Kim J, Han Y, Jeon BH, Cheong HK, Ahn K. Short-term effects of weather and air pollution on atopic dermatitis symptoms in children: a panel study in Korea. PLoS One. 2017; 12:e0175229.

6. Bachert C. Histamine--a major role in allergy? Clin Exp Allergy. 1998; 28:Suppl 6. 15–19.
7. Buddenkotte J, Maurer M, Steinhoff M. Histamine and antihistamines in atopic dermatitis. In : Thurmond RL, editor. Histamine in inflammation. New York: Springer;2010. p. 73–80.
8. Seeman E. Structural basis of growth-related gain and age-related loss of bone strength proceedings of a satellite symposium held on the occasion of the EULAR Congress, Paris, France, June 13, 2008. Rheumatology. 2008; 47:iv2–iv8.

9. Johansson C, Roupe G, Lindstedt G, Mellström D. Bone density, bone markers and bone radiological features in mastocytosis. Age Ageing. 1996; 25:1–7.


10. Folwarczna J, Śliwiński L, Pytlik M, Janas A. Effects of histamine H1, H2 and H3 receptor antagonists on bone mechanical properties in female rats. Bone. 2011; 48:S193.
11. Dobigny C, Saffar JL. H1 and H2 histamine receptors modulate osteoclastic resorption by different pathways: evidence obtained by using receptor antagonists in a rat synchronized resorption model. J Cell Physiol. 1997; 173:10–18.


12. Mandhane SN, Shah JH, Thennati R. Allergic rhinitis: an update on disease, present treatments and future prospects. Int Immunopharmacol. 2011; 11:1646–1662.


13. Kawauchi H, Yanai K, Wang DY, Itahashi K, Okubo K. Antihistamines for allergic rhinitis treatment from the viewpoint of nonsedative properties. Int J Mol Sci. 2019; 20:E213.

14. Luzzi V, Ierardo G, Viscogliosi A, Fabbrizi M, Consoli G, Vozza I, et al. Allergic rhinitis as a possible risk factor for malocclusion: a case-control study in children. Int J Paediatr Dent. 2013; 23:274–278.


15. Pawankar R, Canonica GW, Holgate ST, Lockey RF, Blaiss M. World Allergy Organization (WAO) white book on allergy. Milwaukee, WI: World Allergy Organization;2011.
16. Meh A, Sprogar S, Vaupotic T, Cör A, Drevenšek G, Marc J, et al. Effect of cetirizine, a histamine (H(1)) receptor antagonist, on bone modeling during orthodontic tooth movement in rats. Am J Orthod Dentofacial Orthop. 2011; 139:e323–e329.

17. Parfitt AM, Drezner MK, Glorieux FH, Kanis JA, Malluche H, Meunier PJ, et al. Bone histomorphometry: standardization of nomenclature, symbols, and units. Report of the ASBMR Histomorphometry Nomenclature Committee. J Bone Miner Res. 1987; 2:595–610.


18. Mao JJ, Wang X, Kopher RA. Biomechanics of craniofacial sutures: orthopedic implications. Angle Orthod. 2003; 73:128–135.

19. Kobayashi ET, Hashimoto F, Kobayashi Y, Sakai E, Miyazaki Y, Kamiya T, et al. Force-induced rapid changes in cell fate at midpalatal suture cartilage of growing rats. J Dent Res. 1999; 78:1495–1504.


21. Wu BH, Kou XX, Zhang C, Zhang YM, Cui Z, Wang XD, et al. Stretch force guides finger-like pattern of bone formation in suture. PLoS One. 2017; 12:e0177159.

22. Hou B, Fukai N, Olsen BR. Mechanical force-induced midpalatal suture remodeling in mice. Bone. 2007; 40:1483–1493.



23. Lai RF, Zhou ZY, Chen T. Accelerating bone generation and bone mineralization in the interparietal sutures of rats using an rhBMP-2/ACS composite after rapid expansion. Exp Anim. 2013; 62:189–196.



24. Matuszewska A, Nowak B, Jędrzejuk D, Landwójtowicz M, Sadanowicz E, Sozański T, et al. Effect of long-term administration of ranitidine, a histamine H2 receptor antagonist, on bone metabolism in young growing rats. Pharmacol Rep. 2018; 70:951–954.

25. Biosse-Duplan M, Baroukh B, Dy M, de Vernejoul MC, Saffar JL. Histamine promotes osteoclastogenesis through the differential expression of histamine receptors on osteoclasts and osteoblasts. Am J Pathol. 2009; 174:1426–1434.



26. Lesclous P, Guez D, Saffar JL. Short-term prevention of osteoclastic resorption and osteopenia in ovariectomized rats treated with the H(2) receptor antagonist cimetidine. Bone. 2002; 30:131–136.


27. Yamaura K, Yonekawa T, Nakamura T, Yano S, Ueno K. The histamine H2-receptor antagonist, cimetidine, inhibits the articular osteopenia in rats with adjuvant-induced arthritis by suppressing the osteoclast differentiation induced by histamine. J Pharmacol Sci. 2003; 92:43–49.






 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download