Abstract
Objective
The Alpha stent (CGBio), a new intracranial stent featuring a re-sheathable mesh design with improved wall apposition at the curved segment, was clinically evaluated. We report the 6-month follow-up results from a prospective, single-center study in which the stent was used for coiling of wide-necked distal internal carotid artery (ICA) aneurysms.
Materials and Methods
Between April 2016 and 2018, 50 patients (mean age, 56.5 years, 45 females [90%]) with 54 unruptured distal ICA aneurysms (average diameter: 5.6 ± 1.7 mm) were enrolled. The primary endpoint for effectiveness was successful coil embolization with the Alpha stent, and subsequent complete or near-complete occlusion at the 6-month magnetic resonance angiography assessment. The primary safety endpoint was the absence of serious adverse events (SAEs) up to 6 months from the procedure.
Results
The primary effectiveness endpoint was observed in 94.4% (51/54) aneurysms. In one patient with technical failure, the stent could not be deployed because of parent artery tortuosity; therefore, a different type of stent was used. Of the 53 aneurysms treated with the Alpha stent, complete occlusion was achieved in 64.1% (34/53) cases, and near-complete occlusion was achieved in 32.0% (17/53) cases by the 6-month follow-up. Two cases (3.7%) required retreatment because of major recurrence. In 4% (2/50) patients, SAEs, i.e., retinal artery thromboembolism and corona radiata lacunar infarction, were reported after the procedure.
Stent-assisted coil embolization has become a state-of-the-art method for endovascular treatment of various wide-necked intracranial aneurysms. After the successful introduction of the open-cell design, self-expanding Neuroform stent (the latest version, Neuroform Atlas stent; Stryker Neurovascular, Fremont, CA, USA), another laser-cut self-expanding Enterprise stent with a closed-cell design (the latest version, Enterprise 2 stent; Cerenovus, Raynham, MA, USA) was introduced. More recently, braided self-expanding stents (LVIS and LVIS Jr. stents; Microvention, Tustin, CA, USA) have been gaining popularity.
Understanding the merits and demerits of each stent is necessary for choosing the appropriate stent because a single type of stent may not be suitable for all situations (12). With respect to the cell type, in comparison to closed cells—especially those used in the laser-cut stent—open cells offer good wall apposition. However, since they are not repositionable, these stents require careful deployment. In cases involving coiling of a wide-necked aneurysm at the distal intracranial internal carotid artery (ICA), we frequently experienced limitations because of curvatures present either at the anterior genu portion or in the communicating segment just below the ICA bifurcation.
Recently, a new type of laser-cut, modified closed-cell stent called the Alpha stent (CGBio, Seongnam, Korea) was introduced. It has a characteristic hybrid-type cell design, which aims to improve wall apposition at the curved segment similar to an open-cell stent. However, it is also re-sheathable like a closed-cell stent before complete deployment.
The purpose of this study was to elucidate the initial effectiveness and safety profile as well as the mid-term (6 months) follow-up clinical and angiographic results obtained using the Alpha stent for coil embolization of wide-necked distal ICA aneurysms.
This study was a prospective, single-arm, single-center clinical study that aimed to evaluate the safety and effectiveness of the Alpha stent in coil embolization of distal ICA aneurysms. The study was approved by the Institutional Review Board of our institution, and written informed consent was obtained from each patient.
Detailed inclusion and exclusion criteria are included in the Supplementary Materials. In summary, we included unruptured, wide-necked aneurysms located in the distal ICA as well as saccular aneurysms arising from a parent vessel with a diameter ≥ 3.0 mm and ≤ 4.0 mm, which were considered feasible for stent-assisted coil embolization.
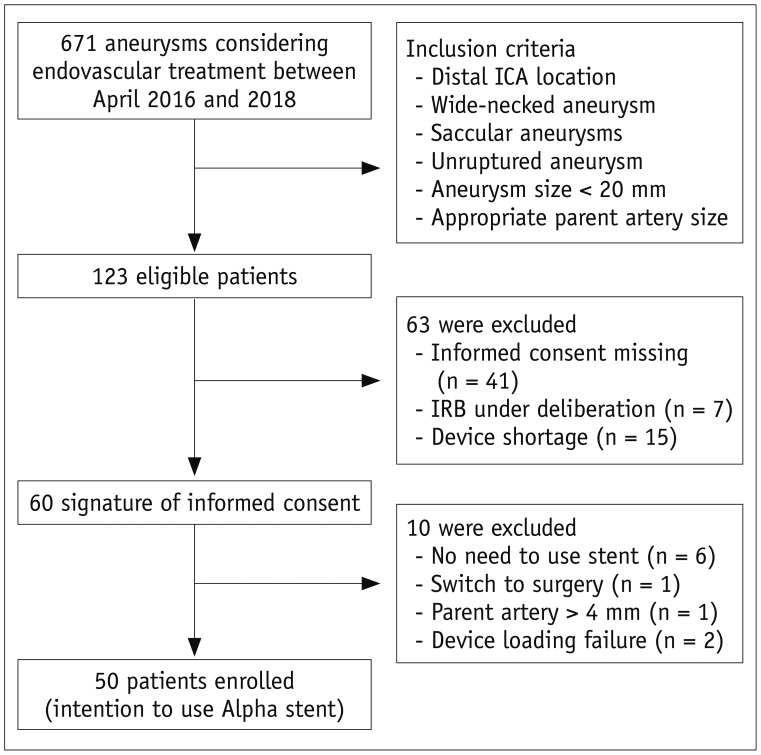
Between April 2016 and April 2018, a total of 60 patients met the initial screening criteria and provided consent (Fig. 1). Ten patients were excluded from the study during the procedures (refer to the Supplementary Material for details). Finally, 50 patients were included in this study. They were 45 women and 5 men, and their mean age was 56.5 ± 9.2 years (range: 34–75 years).
The Alpha stent is a newly developed, self-expanding stent fabricated by laser cutting of a nitinol tube. Unlike the typical laser-cut stents used for aneurysm coiling, which are manufactured by inflating a small-caliber metal tube after laser cutting to the unconstrained diameter of its final shape, the mesh structure of the Alpha stent was created by direct laser cutting of a nitinol metal tube of the unconstrained diameter (5 mm). This was then treated under high temperature to obtain super elasticity. The characteristic feature of this stent is its cell shape, which contains alternating columns of short and long cells (Fig. 2). To increase the longitudinal flexibility and kink resistance of this basically closed-cell type stent, one of the short arms of the smaller cells was removed, resulting in elongated cells. In addition, the stent is fully re-sheathable and repositionable, in theory, until the proximal locking bumper is within the microcatheter. Four tiny platinum rods are attached at each end of the stent as radiopaque markers. The Alpha stent is available in two types according to the length (i.e., 17 mm and 22 mm) with the same fully expanded diameter (5 mm). The stent is deliverable with any kind of microcatheter with a 0.021-inch internal diameter.
All procedures were performed under general anesthesia. All patients were pre-treated with 100 mg of aspirin and 75 mg of clopidogrel daily for 10 days before the procedure. During the procedure, each patient received 50–70 IU/kg of intravenous heparin to obtain an activated clotting time of approximately 250–300 seconds. Two experienced neurointerventionists performed all the procedures in this study. Upon discharge, patients were placed on a 2-month course of dual antiplatelet agents, followed by aspirin monotherapy for at least 4 months.
An Alpha stent was delivered with a 0.021-inch microcatheter (Headway® 21; Microvention, Tustin, CA, USA or Prowler plus®; Codman Neurovascular, Raynham, MA, USA). The strategies used and evaluated for stent deployment in relation to coiling in 53 treated aneurysms were as follows: 1) jailing (n = 33, 62.3%); the microcatheter was “jailed” between the arterial wall and the stent with/without deployment of several coil loops before stent placement, 2) through the struts (n = 8, 15.1%); the microcatheter was introduced into the aneurysm through the struts of the stent, 3) through the struts after jailing (n = 8, 15.1%); the aneurysm was catheterized again through the struts and additional coils were deployed with the same or an additional microcatheter, 4) bailout stenting (n = 3, 5.7%); the coils were introduced before stent deployment, and stent deployment was performed to reverse coil protrusion and/or if the coil mass appeared unstable in some patients in whom coil-only embolization was tried initially, and 5) temporary stenting (n = 1, 1.9%); coiling after partial deployment of the stent (semi-jailing). The stent was re-sheathed and removed when the solid coil mass appeared stable.
To establish a baseline for follow-up imaging, we routinely obtained high-resolution time-of-flight magnetic resonance angiography (MRA) images on post-procedural day 1 and 6 months after the procedure. In order to determine further treatment, digital subtraction angiography (DSA) was recommended when MRA imaging was not feasible or when significant aneurysmal recurrence was suspected. The treatment outcomes were classified into class I (complete 100% occlusion), class II (near-complete occlusion with a small neck remnant showing greater than 95% occlusion), and class III (incomplete occlusion with evidence of residual filling or large aneurysm remnant or lobule showing less than 95% occlusion) (3). A recurrence was defined as any increase in aneurysmal filling at follow-up. Major recurrence included an aneurysmal recurrence of more than 2 mm to provide an appropriate cavity for additional placement of coils (4).
Adverse events (AEs) were defined as undesirable and unintended signs, symptoms, or diseases that developed during the clinical trial regardless of a cause-and-effect relationship with the stent. These included any peri-procedural or post-procedural complications. Serious adverse events (SAEs) included death or life-threatening events, extension of the hospitalization period longer than 3 days or requiring re-admission, or permanent disability. The modified Rankin scale (mRS) score was used to assess functional outcome at the time of discharge and at the 1- and 6-month follow-up visits. Morbidity related to treatment was defined by an mRS score of 3, 4, or 5 (moderate to severe neurological disability).
The primary endpoint for effectiveness was defined as successful coil embolization with the Alpha stent, and satisfactory outcome at the 6-month follow-up angiography (complete or near-complete obliteration in the absence of major recurrence, retreatment, or rupture of the aneurysm). The primary safety endpoint was the absence of SAEs up to 6 months following the procedure.
Technical success of the stent was defined as satisfactory coiling in addition to access to the lesion, successful deployment of the stent over the aneurysm, and no stent loss. We reported stent-related problems, such as re-sheathing problems, expansion problems, displacement of the stent, as well as any AEs that occurred up to 6 months after the procedure.
A total of 50 patients with 54 unruptured, wide-necked, distal ICA aneurysms (average maximum diameter, 5.6 ± 1.7 mm) were enrolled. In four patients, two adjacent aneurysms were covered by one stent. Of these 54 aneurysms, most lesions were paraclinoid ICA aneurysms (94.4%). One aneurysm recurred after a previous coil embolization. Table 1 summarizes aneurysm characteristics and device usage.
Technical success was achieved in 48 of 50 patients (96.0%). In one case, the device could not be deployed at the correct position because of parent vessel tortuosity; thus, a different stent was used. The other technical failure resulted from stent migration during the removal of the pusher wire. The small bump at the pusher wire served as a hook latching the stent strut. The migrated stent could be removed with a micro-snare. Subsequent stent deployment was successful; however, this event was considered a technical failure.
Other device-related problems not associated with technical failure occurred in 10 patients: re-sheathing of the partially deployed stent was required in five patients because of unintended downward displacement of the deployment system during unsheathing of the stent. The stents were successfully re-sheathed and deployed after repositioning of the microcatheter. In three patients, some portions of the stent did not fully open after deployment. This was confirmed on flat-detector computed tomography. Despite this, additional procedures—such as ballooning—were not needed. Successful coiling of the aneurysm was achieved. Mild stent displacement after deployment occurred during subsequent passage of the microcatheter and/or guidewire in two cases; however, the movement did not influence the scaffolding of the aneurysm neck for the coiling.
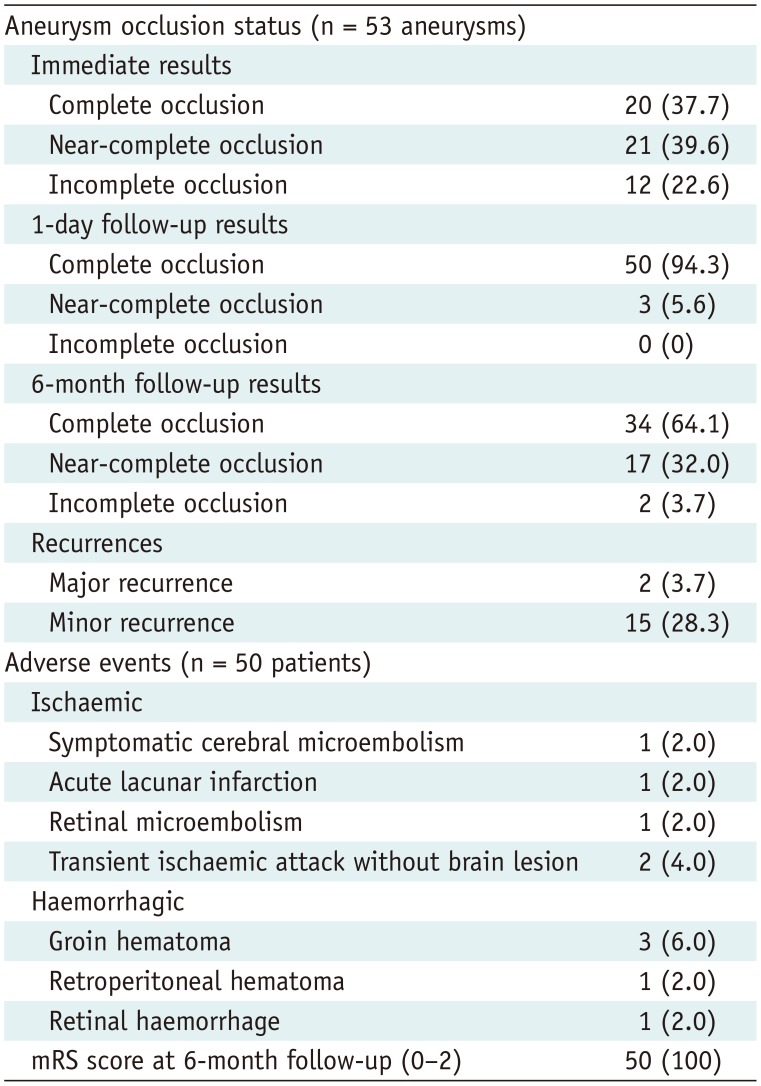
An independent neuroradiologist assessed the final control angiographic occlusion status for all 53 aneurysms (Table 2). Twenty (37.7%) were completely occluded, 21 (39.6%) showed near-complete occlusion, and 12 (22.6%) had incomplete occlusion. MRA on the following day showed complete occlusion in 50 aneurysms (94.3%) and near-complete occlusion in 3 aneurysms (5.6%), but there was no incomplete occlusion. Angiographic data at 6 months were available for all 53 aneurysms. The data showed complete occlusion of 34 aneurysms (64.1%), near-complete occlusion of 17 (32.0%), and incomplete occlusion of 2 (3.7%). Seventeen patients (32.0%) showed aneurysm recurrence; however, major recurrence that needed retreatment was found only in two patients who showed incomplete occlusion at the 6-month follow-up. The primary effectiveness endpoint was achieved in 51 (94.4%) of 54 aneurysms. There were three cases of technical failure: use of a different stent (n = 1) and major recurrence at 6 months (n = 2). A representative example is presented in Figure 3.
SAEs during treatment until the 6-month follow-up period were reported in two patients. One patient (case 40) developed a visual field defect of the right eye after treatment of the ipsilateral paraclinoid ICA aneurysm, suggesting a central retinal artery microembolism. The symptom, visual field defects, did not recover until the 6-month follow-up period. The other patient (case 19) with a left paraclinoid ICA aneurysm developed right arm weakness (grade 4/5) immediately after the procedure. It resulted from a small lacunar infarction in the left corona radiata. This was found on diffusion-weighted MR images. The neurologic symptom had almost improved; however, the patient still complained of weakness of the affected hand at the 6-month follow-up visit.
AEs other than SAEs included symptomatic microembolism (n = 1) that caused a self-limited transient right leg weakness, transient ischemic attack (n = 2) that showed recurrent episodes of hand weakness, groin (n = 3) and retroperitoneal (n = 1) hematomas that did not require any surgical intervention or transfusion, as well as retinal hemorrhage (n = 1) that possibly resulted from the intake of antiplatelet medication. At the 6-month follow-up, 48 out of 50 patients (96%) showed mRS 0, and 2 patients (cases 40 and 19) were assessed as mRS 2 (persistent visual field defects) and mRS 1 (residual mild arm weakness).
With our single-center prospective study, we found that the use of the Alpha stent for coil embolization of unruptured, wide-necked, distal ICA aneurysms was feasible with acceptable safety profiles and mid-term occlusion rates. Because the Alpha stent has a unique mesh design, its clinical availability would increase users' choices of stents for stent-assisted coil embolization, in addition to several other stents in the market.
Given the improvements in device performance and procedural techniques, the use of stents has become technically less demanding. However, understanding the background mechanism of each stent system is important in choosing an appropriate stent, minimizing adverse reactions, and taking full advantage of each stent in various patient situations. We believe that a distal ICA aneurysm is one such specific situation because the lesions tend to have wide necks and dilated and tortuous parent arteries. Such a combination may pose difficulty in the procedures (5). With open-cell-type stents, such as the Neuroform Atlas, exact placement tends to be difficult because of the tortuosity of the target region. Misplacement of the stent might complicate the procedure, because re-sheathing and repositioning of the stent are not possible once deployment is initiated. Even after successful placement of the stent, re-crossing of the stent mesh is not easy in cases using the stent-through technique. In contrast, with closed-cell-type stents, such as the Enterprise 2 stent or the Solitaire AB stent (Medtronic, Minneapolis, MN, USA), neck coverage is less reliable and wall apposition, especially at the curved region, tends to be inappropriate. Even with braided-type stents such as the LVIS stent or the Leo stent (Balt, Montmorency, France), the deployment process tends to be complicated because the outcome of deployment is highly operator-dependent and re-crossing of the mesh is challenging and sometimes not possible because of a relatively small cell size.
The Alpha stent is a closed-cell-type, laser-cut stent with flared ends that is basically similar in structure to the Enterprise stent. The difference, as shown in Figure 2, is that the Alpha stent has longitudinally arranged, elongated rectangular smaller cells in between the leaf-shaped, larger cells. It was designed such that the width of the elongated cell could increase in the convex side and decrease in the concave side to ensure better performance of the stent in the curvature. With accumulation of operators' experience of the Alpha stent, we noted good wall apposition of the stent mesh, even at the curve regions such as in the anterior genu of the ICA. Further analysis of the mechanical and physical properties of the stent and exact cell shape change pattern will be helpful in understanding its exact mechanism.
The published rates of neurologic complications were 2.7–14.8% in patients treated with stent-assisted coiling for wide-necked aneurysms, with varying proportions (5.5–29.6%) of ruptured aneurysms (678910). The morbidity and mortality rates ranged from 0% to 3% and 0% to 3.3%, respectively. In our study, periprocedural neurologic complications occurred in five patients (10%), but there was no permanent morbidity and mortality at the 6-month follow-up. The safety outcome of our study is comparable to those of previous studies, even though ruptured aneurysms were not included in our study.
With respect to aneurysmal occlusion on the 6-month angiography image, our study demonstrated complete occlusion in 64.1% (34/53) and near-complete occlusion in 32.0% (17/53) of the cases. For the Neuroform Atlas stent, Ten Brinck et al. (7) and Ulfert et al. (6) reported 53.8% and 93% complete occlusion and 15.4% and 7.0% of residual neck at 6 months, respectively. For the Enterprise stent, Wakhloo et al. (11) reported up to 80% complete occlusion over a mean period of 11.9 months. For the LVIS device, Iosif et al. (8) and Fiorella et al. (9) reported complete occlusion (Raymond class I or 100% occlusion) rates of 91% and 75% at 6 months, respectively, while Shankar et al. (10) reported 68% at a median of 12 months. The occlusion rates were 78–100% when including cases with near-complete occlusion in these studies. Our study showed comparable outcomes to other devices in terms of favorable occlusion (Raymond class I/II or > 95% occlusion).
Recent studies of stent-assisted coil embolization have reported the recanalization rates of LVIS (2.5–5.1%) (81213), Neuroform Atlas (0–15.4%) (67), and Enterprise (10.7%) (13), which varied substantially depending on the devices and studies. Major recanalization that needed retreatment was documented in two cases (3.7%), while minor recanalization was documented in 15 cases (28.3%) in our study. The overall recanalization rate of 31.4% was very high in our series, possibly because we regarded any growth of aneurysm filling as a recanalization, regardless of the degree of change in occlusion. Most of these instances were minimal and did not require retreatment.
This study has several limitations that should be emphasized. First, a 6-month follow-up period may not be sufficient to assess aneurysm occlusion or recurrence rate. However, since the purpose of our study was to evaluate the initial safety and effectiveness of the newly developed stent, we believed that our study provided sufficient evidence regarding the initial results obtained with the Alpha stent. In addition, the average overall follow-up duration was 13.4 months, and no significant safety or recurrence problems were found over 6 months. Second, the lack of DSA results in all patients may be another limitation. Because of our current practice protocol, which typically lacks routine DSA imaging follow-up after endovascular coiling, it was practically impossible to perform DSA follow-up routinely for this clinical study. As was previously reported (14), we believe that MRA could be the standard follow-up imaging tool after coiling of cerebral aneurysms. Third, as this study had a single-arm design, we could not provide comparative results with other stents. Fourth, although we tried to enroll consecutive patients who satisfied the inclusion criteria, there may be a potential selection bias. Indeed, more than half of the initial eligible patients were excluded from the study as we have described in Figure 1. Finally, as we only included ICA aneurysms and most of them were located at the side wall, our results might not be generalizable to other aneurysms.
In conclusion, for endovascular treatment of unruptured, wide-necked, distal ICA aneurysms, coil embolization using the newly developed Alpha stent showed excellent procedural and mid-term clinical follow-up results in terms of effectiveness and safety.
Acknowledgments
We would like to thank Ga Young Lee, Seon Moon Hwang, and Seunghye Hong who helped us a lot in supporting this study.
Notes
References
1. Dholakia RJ, Kappel AD, Pagano A, Woo HH, Lieber BB, Fiorella DJ, et al. In vitro angiographic comparison of the flow-diversion performance of five neurovascular stents. Interv Neuroradiol. 2018; 24:150–161. PMID: 29239685.
2. Cho SH, Jo WI, Jo YE, Yang KH, Park JC, Lee DH. Bench-top comparison of physical properties of 4 commercially-available self-expanding intracranial stents. Neurointervention. 2017; 12:31–39. PMID: 28316867.

3. Kole MK, Pelz DM, Kalapos P, Lee DH, Gulka IB, Lownie SP. Endovascular coil embolization of intracranial aneurysms: important factors related to rates and outcomes of incomplete occlusion. J Neurosurg. 2005; 102:607–615. PMID: 15871501.

4. Raymond J, Guilbert F, Weill A, Georganos SA, Juravsky L, Lambert A, et al. Long-term angiographic recurrences after selective endovascular treatment of aneurysms with detachable coils. Stroke. 2003; 34:1398–1403. PMID: 12775880.

5. Lee JW, Woo JM, Lim OK, Jo YE, Kim JK, Kim ES, et al. Enlarged parent artery lumen at aneurysmal-neck segment in wide-necked distal internal carotid artery aneurysms. Neurointervention. 2015; 10:82–88. PMID: 26389011.

6. Ulfert C, Pham M, Sonnberger M, Amaya F, Trenkler J, Bendszus M, et al. The Neuroform Atlas stent to assist coil embolization of intracranial aneurysms: a multicentre experience. J Neurointerv Surg. 2018; 10:1192–1196. PMID: 29678886.

7. Ten Brinck MFM, de Vries J, Bartels RHMA, Grotenhuis JA, Boogaarts HD. NeuroForm Atlas stent-assisted coiling: preliminary results. Neurosurgery. 2019; 84:179–189. PMID: 29579261.

8. Iosif C, Piotin M, Saleme S, Barreau X, Sedat J, Chau Y, et al. TRAIL Investigators. Safety and effectiveness of the Low Profile Visualized Intraluminal Support (LVIS and LVIS Jr) devices in the endovascular treatment of intracranial aneurysms: results of the TRAIL multicenter observational study. J Neurointerv Surg. 2018; 10:675–681. PMID: 29175829.

9. Fiorella D, Boulos A, Turk AS, Siddiqui AH, Arthur AS, Diaz O, et al. LVIS investigators. The safety and effectiveness of the LVIS stent system for the treatment of wide-necked cerebral aneurysms: final results of the pivotal US LVIS trial. J Neurointerv Surg. 2019; 11:357–361. PMID: 30297543.

10. Shankar JJS, Quateen A, Weill A, Tampieri D, Del Pilar Cortes M, Fahed R, et al. Canadian Registry of LVIS Jr for Treatment of Intracranial Aneurysms (CaRLA). J Neurointerv Surg. 2017; 9:849–853. PMID: 27543629.

11. Wakhloo AK, Linfante I, Silva CF, Samaniego EA, Dabus G, Etezadi V, et al. Closed-cell stent for coil embolization of intracranial aneurysms: clinical and angiographic results. AJNR Am J Neuroradiol. 2012; 33:1651–1656. PMID: 22492570.

12. Zhang X, Zhong J, Gao H, Xu F, Bambakidis NC. Endovascular treatment of intracranial aneurysms with the LVIS device: a systematic review. J Neurointerv Surg. 2017; 9:553–557. PMID: 27206450.

13. Ge H, Lv X, Yang X, He H, Jin H, Li Y. LVIS stent versus enterprise stent for the treatment of unruptured intracranial aneurysms. World Neurosurg. 2016; 91:365–370. PMID: 27113398.

14. Cho YD, Kim KM, Lee WJ, Sohn CH, Kang HS, Kim JE, et al. Time-of-flight magnetic resonance angiography for follow-up of coil embolization with enterprise stent for intracranial aneurysm: usefulness of source images. Korean J Radiol. 2014; 15:161–168. PMID: 24497808.
Supplementary Materials
The Data Supplement is available with this article at https://doi.org/10.3348/kjr.2019.0188.
Fig. 2
Photograph of Alpha stent in unconstrained status and schematic diagram showing arrangement of main fish-scale-like closed cells and intervening elongated small cells with very narrow width (arrows).
Although meshes are composed of closed cells, elongated narrow cells provide better conformability of stent even in curve regions.
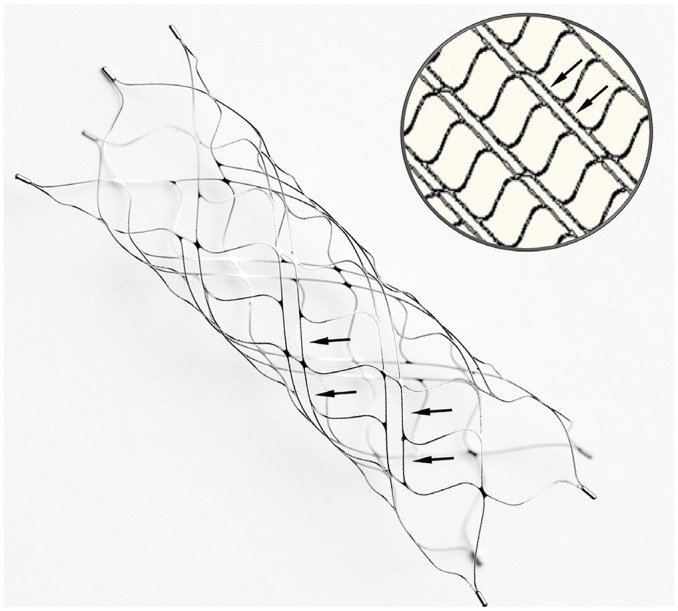
Fig. 3
Representative case of stent-assisted coil embolization in patient with tortuous ICA.
A. 3D rotational angiogram of left ICA shows 8-mm multilobulated wide-necked aneurysm (arrow) at ophthalmic segment of left ICA. B. On scout image, radiopaque tip of lead wire (arrowhead), four distal radiopaque markers (thin arrow), and stent struts are well visualized. Proximal markers (thick arrow) are still in catheter. C. Stent was completely expanded even in curvature of proximal segment, which was confirmed on cone-beam CT. D. Successive coiling was performed through struts and near-complete occlusion was achieved on final angiography. E. On magnetic resonance angiography performed on next day, no residual aneurysm was found. Parent vessel (arrows) over stent was poorly visualized due to metallic artefact. F. 3D digital subtraction angiography image obtained on 3-month follow-up demonstrated complete occlusion of aneurysm and patent parent vessel. 3D = three-dimensional
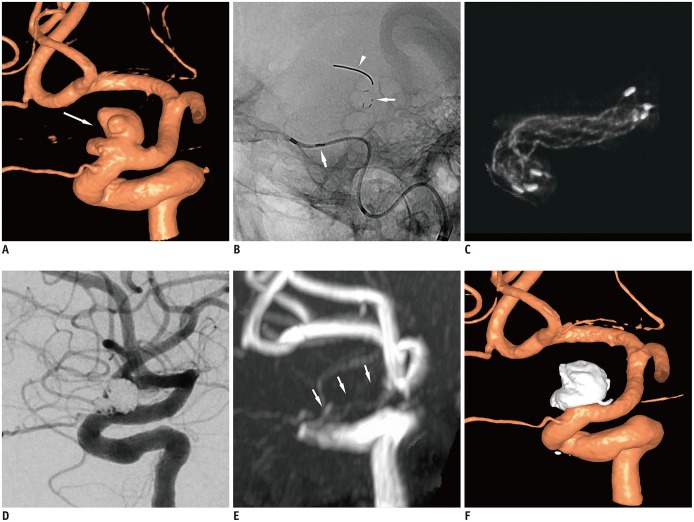
Table 1
Aneurysm and Procedure Characteristics
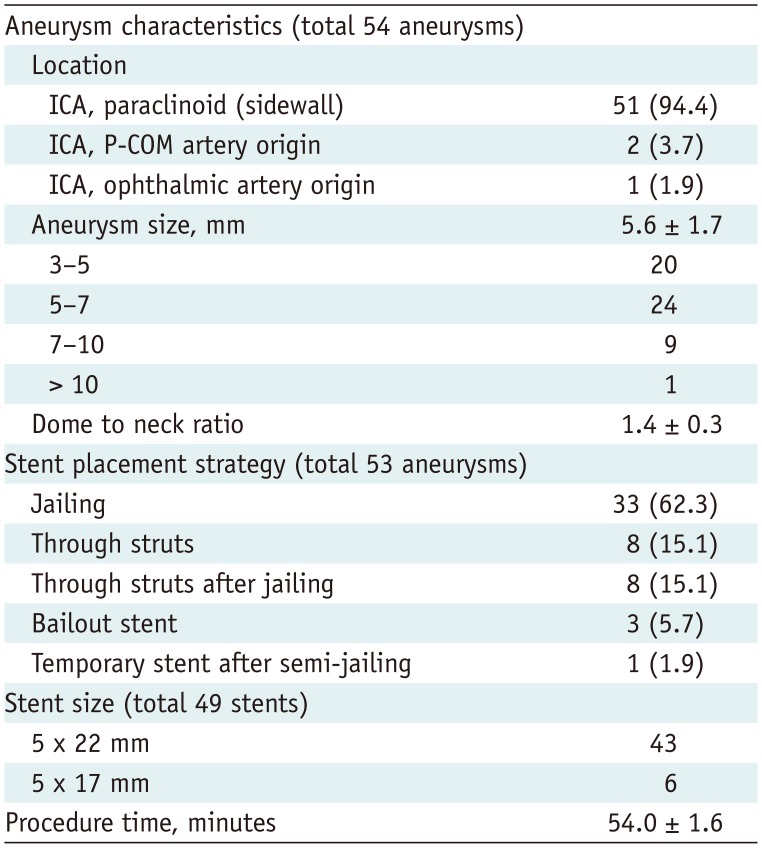
Table 2
Angiographic and Clinical Outcomes




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download