1. Lee H, Kim TH, Baek YS, Uhm JS, Pak HN, Lee MH, et al. The trends of atrial fibrillation-related hospital visit and cost, treatment pattern and mortality in Korea: 10-year nationwide sample cohort data. Korean Circ J. 2017; 47:56–64. PMID:
28154592.

2. Kim TH, Yang PS, Uhm JS, Kim JY, Pak HN, Lee MH, et al. CHA
2DS
2-VASc score (congestive heart failure, hypertension, age ≥75 [doubled], diabetes mellitus, prior stroke or transient ischemic attack [doubled], vascular disease, age 65-74, female) for stroke in Asian patients with atrial fibrillation: a Korean nationwide sample cohort study. Stroke. 2017; 48:1524–1530. PMID:
28455320.
3. Kim D, Yang PS, Jang E, Yu HT, Kim TH, Uhm JS, et al. 10-year nationwide trends of the incidence, prevalence, and adverse outcomes of non-valvular atrial fibrillation nationwide health insurance data covering the entire Korean population. Am Heart J. 2018; 202:20–26. PMID:
29802976.

4. Kim D, Yang PS, Jang E, Yu HT, Kim TH, Uhm JS, et al. Increasing trends in hospital care burden of atrial fibrillation in Korea, 2006 through 2015. Heart. 2018; 104:2010–2017. PMID:
29666179.

5. Joung B, Lee JM, Lee KH, Kim TH, Choi EK, Lim WH, et al. 2018 Korean guideline of atrial fibrillation management. Korean Circ J. 2018; 48:1033–1080. PMID:
30403013.

6. Yoon M, Yang PS, Jang E, Yu HT, Kim TH, Uhm JS, et al. Dynamic changes of CHA2DS2-VASc score and the risk of ischaemic stroke in Asian patients with atrial fibrillation: a nationwide cohort study. Thromb Haemost. 2018; 118:1296–1304. PMID:
29723875.

7. Kim KE, Yang PS, Jang E, Kim S, Joung B. Antithrombotic medication and the risk of vitreous hemorrhage in atrial fibrillation: Korean National Health Insurance Service National Cohort. Yonsei Med J. 2019; 60:65–72. PMID:
30554492.

8. Hart RG, Pearce LA, Aguilar MI. Meta-analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med. 2007; 146:857–867. PMID:
17577005.

9. van Walraven C, Hart RG, Singer DE, Laupacis A, Connolly S, Petersen P, et al. Oral anticoagulants vs aspirin in nonvalvular atrial fibrillation: an individual patient meta-analysis. JAMA. 2002; 288:2441–2448. PMID:
12435257.
10. Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009; 361:1139–1151. PMID:
19717844.

11. Lee SR, Lee YS, Park JS, Cha MJ, Kim TH, Park J, et al. Label adherence for non-vitamin K antagonist oral anticoagulants in a prospective cohort of Asian patients with atrial fibrillation. Yonsei Med J. 2019; 60:277–284. PMID:
30799590.

12. Nelson WW, Song X, Coleman CI, Thomson E, Smith DM, Damaraju CV, et al. Medication persistence and discontinuation of rivaroxaban versus warfarin among patients with non-valvular atrial fibrillation. Curr Med Res Opin. 2014; 30:2461–2469. PMID:
24926732.

13. Kirchhof P, Benussi S, Kotecha D, Ahlsson A, Atar D, Casadei B, et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J. 2016; 37:2893–2962. PMID:
27567408.

14. Steffel J, Verhamme P, Potpara TS, Albaladejo P, Antz M, Desteghe L, et al. The 2018 European Heart Rhythm Association Practical Guide on the use of non-vitamin K antagonist oral anticoagulants in patients with atrial fibrillation: executive summary. Europace. 2018; 20:1231–1242. PMID:
29562331.

15. Lip GYH, Collet JP, Haude M, Byrne R, Chung EH, Fauchier L, et al. 2018 Joint European consensus document on the management of antithrombotic therapy in atrial fibrillation patients presenting with acute coronary syndrome and/or undergoing percutaneous cardiovascular interventions: a joint consensus document of the European Heart Rhythm Association (EHRA), European Society of Cardiology Working Group on Thrombosis, European Association of Percutaneous Cardiovascular Interventions (EAPCI), and European Association of Acute Cardiac Care (ACCA) endorsed by the Heart Rhythm Society (HRS), Asia-Pacific Heart Rhythm Society (APHRS), Latin America Heart Rhythm Society (LAHRS), and Cardiac Arrhythmia Society of Southern Africa (CASSA). Europace. 2019; 21:192–193. PMID:
30052888.

16. Lip GY, Windecker S, Huber K, Kirchhof P, Marin F, Ten Berg JM, et al. Management of antithrombotic therapy in atrial fibrillation patients presenting with acute coronary syndrome and/or undergoing percutaneous coronary or valve interventions: a joint consensus document of the European Society of Cardiology Working Group on Thrombosis, European Heart Rhythm Association (EHRA), European Association of Percutaneous Cardiovascular Interventions (EAPCI) and European Association of Acute Cardiac Care (ACCA) endorsed by the Heart Rhythm Society (HRS) and Asia-Pacific Heart Rhythm Society (APHRS). Eur Heart J. 2014; 35:3155–3179. PMID:
25154388.
17. Kim H, Kim TH, Cha MJ, Lee JM, Park J, Park JK, et al. A prospective survey of atrial fibrillation management for real-world guideline adherence: COmparison study of Drugs for symptom control and complication prEvention of Atrial Fibrillation (CODE-AF) Registry. Korean Circ J. 2017; 47:877–887. PMID:
29171211.

18. January CT, Wann LS, Alpert JS, Calkins H, Cigarroa JE, Cleveland JC Jr, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the Heart Rhythm Society. Circulation. 2014; 130:2071–2104. PMID:
24682348.

19. SayXMLLink_XYZn B, Okutucu S, Yılmaz MB, Özdemir K, Aydınlar A, S¸ahin DY, et al. Antithrombotic treatment patterns and stroke prevention in patients with atrial fibrillation in TURKEY: inferences from GARFIELD-AF registry. Anatol J Cardiol. 2019; 21:272–280. PMID:
31062761.

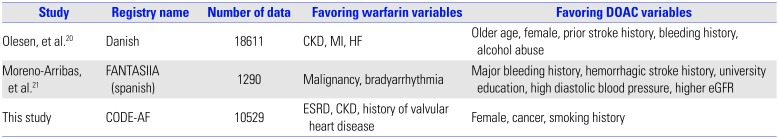
20. Olesen JB, Sørensen R, Hansen ML, Lamberts M, Weeke P, Mikkelsen AP, et al. Non-vitamin K antagonist oral anticoagulation agents in anticoagulant naïve atrial fibrillation patients: Danish nationwide descriptive data 2011-2013. Europace. 2015; 17:187–193. PMID:
25236181.
21. Moreno-Arribas J, Bertomeu-González V, Anguita-Sanchez M, Cequier Á, Muñiz J, Castillo J, et al. Choice of new oral anticoagulant agents versus vitamin K antagonists in atrial fibrillation: FANTASIIA Study. J Cardiovasc Pharmacol Ther. 2016; 21:150–156. PMID:
26229096.
22. Lauffenburger JC, Farley JF, Gehi AK, Rhoney DH, Brookhart MA, Fang G. Factors driving anticoagulant selection in patients with atrial fibrillation in the United States. Am J Cardiol. 2015; 115:1095–1101. PMID:
25724781.

23. Camm AJ, Lip GY, De Caterina R, Savelieva I, Atar D, Hohnloser SH, et al. 2012 focused update of the ESC Guidelines for the management of atrial fibrillation: an update of the 2010 ESC Guidelines for the management of atrial fibrillation--developed with the special contribution of the European Heart Rhythm Association. Europace. 2012; 14:1385–1413. PMID:
22923145.
24. Jung H, Yang PS, Jang E, Yu HT, Kim TH, Uhm JS, et al. Effectiveness and safety of non-vitamin K antagonist oral anticoagulants in patients with atrial fibrillation with hypertrophic cardiomyopathy: a nationwide cohort study. Chest. 2019; 155:354–363. PMID:
30472021.
25. Verhoef TI, Redekop WK, Hasrat F, de Boer A, Maitland-van der Zee AH. Cost effectiveness of new oral anticoagulants for stroke prevention in patients with atrial fibrillation in two different European healthcare settings. Am J Cardiovasc Drugs. 2014; 14:451–462. PMID:
25326294.

26. Sandhu RK, Bakal JA, Ezekowitz JA, McAlister FA. Risk stratification schemes, anticoagulation use and outcomes: the risk--treatment paradox in patients with newly diagnosed non-valvular atrial fibrillation. Heart. 2011; 97:2046–2050. PMID:
22076011.

27. Bernard A, Fauchier L, Pellegrin C, Clementy N, Saint Etienne C, Banerjee A, et al. Anticoagulation in patients with atrial fibrillation undergoing coronary stent implantation. Thromb Haemost. 2013; 110:560–568. PMID:
23846210.

28. Azoulay L, Dell’Aniello S, Simon T, Renoux C, Suissa S. The concurrent use of antithrombotic therapies and the risk of bleeding in patients with atrial fibrillation. Thromb Haemost. 2013; 109:431–439. PMID:
23306435.

29. Cannon CP, Bhatt DL, Oldgren J, Lip GYH, Ellis SG, Kimura T, et al. Dual antithrombotic therapy with dabigatran after PCI in atrial fibrillation. N Engl J Med. 2017; 377:1513–1524. PMID:
28844193.

30. Lopes RD, Heizer G, Aronson R, Vora AN, Massaro T, Mehran R, et al. Antithrombotic therapy after acute coronary syndrome or PCI in atrial fibrillation. N Engl J Med. 2019; 380:1509–1524. PMID:
30883055.

31. January CT, Wann LS, Calkins H, Chen LY, Cigarroa JE, Cleveland JC Jr, et al. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS Guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2019; 74:104–132. PMID:
30703431.
32. Neumann FJ, Sousa-Uva M, Ahlsson A, Alfonso F, Banning AP, Benedetto U, et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur Heart J. 2019; 40:87–165. PMID:
30165437.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download