Abstract
Trigeminal neuralgia (TN) is a chronic disorder of the trigeminal nerve characterized by repeated electrical shock-like sensations on one side of the face. It can cause severe pain in the face and disrupt or impair quality of life in patients. Options for the management of TN consist of pharmacological and surgical treatments, including Gamma Knife radiosurgery (GKRS). GKRS has been used for TN for a long time because of its low rate of complications and high success rate. Moreover, GKRS can be of use for drug-resistant TN patients who are poor surgical candidates due to medical comorbidities, patients of older age, or patients who refuse invasive therapy. We reviewed the rationale, effects, safety, and current treatment policies of GKRS for TN in view of our institution's results and a review of the literature to date.
Trigeminal neuralgia (TN) is defined by the International Headache Society as “recurrent unilateral brief electric shock-like pains, abrupt in onset and termination, limited to the distribution of one or more divisions of the trigeminal nerve and triggered by innocuous stimuli.”1 It is characterized by the presence of a trigger zone, no objective neurological deficit, and no other identified causes of facial pain; it can cause severe pain and disrupt or impair quality of life in patients.2
Treatments for TN consist of pharmacological treatments, such as that with carbamazepine; surgical treatments, including open surgery and percutaneous procedures; and radiosurgery, including gamma knife radiosurgery (GKRS). Historically, GKRS was introduced as a treatment for TN. The Swedish neurosurgeon Lars Leksell began treating TN patients in 1951 using a prototype guiding device linked to a dental X-ray machine.3 Lindquist reported on the progress of TN patients who had undergone GKRS in 1991, and several studies have documented the safety and efficacy of GKRS, including long-term results, for TN.456 Other techniques of radiosurgery, such as cyber knife surgery and linear accelerator, were introduced in the 2000s and have shown effectiveness, compared to other surgical treatments.78
Several modifications have been made to the treatment regimens for GKRS over the past few decades. We reviewed the rationale, effects, safety, and current treatment policies of GKRS for TN in view of our institution's results and a review of the literature to date.
The pathogenesis of TN is not fully understood, and its exact pathophysiology remains controversial. However, in general, it is described by a mixed peripheral and central mechanism.9 Neurovascular conflict is the most accepted theory, as it is related to TN in a vast majority of cases.10 Chronic nerve compression results in demyelination of trigeminal sensory fibers within the proximal nerve root, with progressive axonal degeneration.11 Demyelination can lead to ephaptic transmission, and the reentry mechanism causes an amplification of sensory inputs.1012 Meanwhile, nerve injury leads to a release of mediators that sensitize peripheral nerve terminations, resulting in neurochemical and phenotypic changes and increased excitability of trigeminal neurons and trigeminal nuclei (central sensitization).11 Ultrastructural and biochemical changes in axons and myelin are seen in the root, the Gasserian ganglion, or both.13
According to the International Classification of Headache Disorders, 3rd edition (ICHD-3), TN can be diagnosed when recurrent paroxysms of unilateral facial pain of a severely intense, electric shock-like, shooting, stabbing, or sharp nature occurs in one or more divisions of the trigeminal nerve.1 It should not radiate beyond the territory of the trigeminal nerve, should last between 1 second and 2 minutes, and should be precipitated by innocuous stimuli within the affected trigeminal distribution. TN is divided into the following three categories according to its cause: classical TN, secondary TN, and idiopathic TN. Classical TN refers to cases without an apparent cause other than neurovascular compression. When underlying diseases are present, such as a tumor in the cerebellopontine angle, arteriovenous malformation, or multiple sclerosis, secondary TN is diagnosed. Idiopathic TN could be diagnosed in cases where neither an electrophysiological test nor magnetic resonance images show significant abnormalities. Besides the ICHD classification, the Burchiel classification is also used to categorize TN and related facial pain syndromes using the characteristics of pain.14 The classification is based on the patient's history and is shown in Table 1.
Treatments for TN are divided into the following three categories: pharmacological treatments; surgical treatments, including microvascular decompression (MVD) and percutaneous procedures, such as radiofrequency rhizotomy (RFR), balloon microcompression, and glycerol injection; and stereotactic radiosurgery.
The first therapeutic line is pharmacological treatment with carbamazepine. It is the only drug shown in a random-controlled trial to reduce the intensity and frequency of attacks, and it provides significant pain control in 80–85% of patients.15 Nevertheless, its efficacy can decrease, and in the long term, many patients become drug-resistant.16 Oxcarbazepine, baclofen, lamotrigine, and pimozide can also be used.17
Surgical treatment is the second therapeutic line and includes open surgery, percutaneous surgery, and radiosurgery. It is used in patients with medically intractable pain or those who suffer from side effects related to medication. MVD alleviates the underlying cause of a compressed trigeminal nerve root through exploration of the posterior fossa. It is considered a reference treatment modality, as it provides pain relief for approximately 90% of patients and has long-term effects (68–88% after 5 years and 61–88% after 10 years).1819 Therefore, MVD should be considered as the first surgical treatment modality for young patients with an obvious neurovascular conflict.20 However, major complications of surgery can include hearing loss, cerebrospinal fluid leakage, infection, hemorrhage, and brainstem infarction.1821
Percutaneous procedures are ablative techniques performed at the level of the Gasserian ganglion and are mechanistically based on physical, thermic, and chemical actions. Percutaneous procedures show a high initial success rate; however, over time, the recurrence rate is higher than that of MVD.22 The initial success rate for balloon microcompression was reported at 82–99%, and the median pain-free time was 20 months.2324 For glycerol injection, the initial success rate and the median pain-free time were 73–96% and 21 months, respectively.2425 For RFR, the initial success rate was 78.8–100%, and the probabilities of remaining pain-free 1, 2, and 11 years after the procedure were 65%, 49%, and 26%, respectively.262728 These ablative procedures pose a risk of hypesthesia, dysesthesia, severe facial numbness, corneal keratitis, and masseter muscle weakness.2930
Lastly, GKRS can be used for drug-resistant TN patients who are poor surgical candidates due to medical comorbidities or age or for those who refuse invasive therapy, especially in the absence of other primary indications, such as neurovascular conflict. GKRS has a very high rate of pain relief with minimal complications, showing a gradual decline in the complication rate due to advancement in imaging modalities.22 Burchiel's type 1 and type 2 TN are the most common indications of GKRS. GKRS may also be used in selected cases of multiple sclerosis-related and post-herpetic TN and in some cases of atypical facial pain.3132
A total of 235 patients underwent GKRS for TN between February 1996 and September 2018. The indications for GKRS included failure of pharmacological treatment, significant adverse effects from medication, and failure of prior surgical treatment. Of these patients, 157 had charts and a follow-up period of more than 1 year. Of these patients, except for those with TN related to tumors and multiple sclerosis, 142 patients were reviewed (Fig. 1). Patient characteristics are shown in Table 2. GKRS was performed with a Gamma Knife (Leksell Gamma Knife, Elekta Instruments, Atlanta, GA, USA). Seven patients were treated with the Gamma Knife Model B, 28 patients with the Gamma Knife Model C, and 107 patients with the Perfexion Gamma Knife.
GKRS treatment was planned using T2-weighted magnetic resonance images with a slice thickness of 1 mm and constructive interference in steady-state images with 0.5-mm axial slices obtained with the stereotactic frame fixed to the head under local anesthesia. A single 4-mm isocenter with two or three beam blocking was used for treatment. It was positioned to cover the trigeminal root entry zone (REZ). After February 2005, planning was done such that a 15-Gy isodose line invaded less than 5% of the brainstem. The dose used varied from 60 to 90 Gy.
Pain from TN was assessed using Barrow Neurologic Institute (BNI) pain scores. BNI scores of I–IIIb were considered as indicative of successful treatment, whereas BNI pain scores of IV and V were considered reflective of unsuccessful treatment. Following improvements in pain after GKRS, any worsening of the pain (even if the level of pain was milder than that present prior to treatment) was considered as recurrence.
The median follow-up period was 37 months (12–250 months, 54.35±49.51 months). The median dose of gamma rays was 85 Gy (60–90 Gy, 84.23±3.743 Gy). After GKRS, 93.7% of patients (133/142) experienced successful treatment (Fig. 2). Of these, recurrence of pain occurred in 50 patients (37.6%, 50/133), and the median time to recurrence was 29 months (2–166 months, 42.26±38.08 months). Among patients who experienced recurrence, six (12%, 6/50) needed additional surgery or procedures, and the rest were controlled by medication. Complications were reported in a total of 25 patients (17.6%, 25/142) and included facial hypesthesia in 19 patients (13.4%), dysesthesia in 2 patients (1.4%), and dry eye syndrome in 4 patients (2.8%) (Table 3). Univariate comparisons were performed using the independent t-test and one-way ANOVA test. There were no statistically significant correlations between the prior procedure and complications (p=0.068) or between the types of prior procedures and complications (p=0.705). There were also no statistically significant relationships between dose and successful treatment, recurrence, and complications under significance of p<0.05.
The rationale for achieving pain relief after GKRS is related to focal axonal degeneration of the trigeminal nerve associated with nociceptive sensibility, destruction of ionic channels, and electrophysiological blocking initially after nerve irradiation.3334 Additionally, a delayed radiation effect with axonal degeneration has been proposed, as some patients experience a late response after GKRS.35 In the literature, the latency to pain relief was 15 to 78 days on average, and the maximum time to pain relief was 6 months after treatment.29
Table 4 lists studies with more than 100 patients who underwent GKRS for TN (Fig. 3). GKRS showed high rates of pain relief, ranging from 70–98% in large studies of over 100 patients (Fig. 4). Pain control was achieved in 69–85% of cases at 1 year, 59% at 2 years, and 38–52% at 5 years.3536 Regarding long-term results, rates of pain control maintenance were 32–59.7% at 7 years and 30–45.3% at 10 years.5637 Although follow-up periods vary among the studies, making direct comparison difficult, the average pain free duration was 7–58 months.353839 The mean recurrence rate was 26.9%, with a range of 3.3–45.1% (Fig. 5). In comparison, our data showed a higher recurrence rate than the average. This may be due to a difference in follow-up periods, because in our study, some patients experienced recurrent pain after approximately 14 years.
Complications due to GKRS are uncommon, with hypesthesia being the most common adverse event. The incidence of hypesthesia is reported to be up to 2.7–55.0%, with a mean occurrence rate of 22.55%, slightly higher than our result (Table 4). Similar to the latency period for GKRS to be effective, there may also be a latency period between the procedure and the development of complications. The mean time to hypesthesia occurrence ranged from 6 to 36 months.29
Other complications include dysesthesia, deafferentation pain, dysgeusia, dry eye syndrome, keratitis, hearing impairment, and masticator weakness.4142 Rarely, significant injury to adjacent vasculature due to high doses have been reported.43 Terms used in each article related to complications had a lack of precision; hence, there may be differences in the rate of complications. Studies have reported dysesthesia in 0–16%, paresthesia in 0–13%, deafferentation pain in 0–3.3%, dry eye syndrome in 0–22.4%, and keratitis in 0–7%.44454647
Complications were not associated with the prior procedure in previous studies, which is consistent with our results.484950 This may be due to some differences in the mechanisms of complications in destructive procedures, such as GKRS, versus nondestructive procedures, such as MVD. Also, in MVD and other destructive procedures, such as RFR, the duration of complications is short, which does not seem to affect complications in a subsequent surgery. However, in the case of radiosurgery, there is a report that previous radiosurgery affects complications because there is a latency period of radiosurgery.49
Several studies have shown that complication rates are associated with the target selection, the length of the irradiated nerve, and the dose.425152 Our study showed a complication rate similar to that in previous studies, and complications due to GKRS do not appear to be statistically related to the high dose used.
Due to the low incidence of complications associated with GKRS, rather than other procedures, repeat GKRS may be considered for pain recurrence. Repeat GKRS appears to have a similar efficacy to initial GKRS for TN, with rates of complete and partial pain relief ranging from 78% to 85%.515354 There is a report that prior GKRS is associated with bothersome facial numbness after GKRS. The main complications after repeat GKRS are new facial sensory symptoms caused by partial trigeminal nerve injury, seen in 11–69% of patients.545556
Therefore, to reduce complications due to repeat GKRS, decreasing the dose of the second GKRS would be helpful, as would moving the target to not overlap with the previous target, so that a previously treated nerve is not exposed to a high dose of radiation.54
When GKRS was first used for TN, Leksell3 targeted the Gasserian ganglion. After that, Lindquist, et al.4 reported the results of GKRS targeting the Gasserian ganglion in 1991 and then stated the results of treatment of the trigeminal REZ, where peripheral myelination with Schwann cells transits to central myelination with oligodendrocytes. Rand, et al.57 reported the results of the GKRS targeting the retrogasserian area, anterior to REZ, in 1993. Since then, both the REZ and retrogasserian area have been used to treat TN with GKRS (Fig. 6).
There is still controversy regarding which target is better. The major difference between the targets is the dose received by the REZ and the brainstem. However, the effect and complications related to the target and the dose are not established. Several studies have compared the complications and effects between anterior and posterior targets.585960 They reported that an anterior target has lower complications than a posterior target, showing similar or better pain relief. However, since there is a difference between doses among studies and targets and the appropriate dose has not yet been established for the target, it is difficult to directly determine which target is better.
Determination of the treatment dose may vary according to the target. However, studies have shown that a dose of 70 Gy failed to control pain in 100% and that radiation below 70 Gy had little effect on the structure of the trigeminal nerve.6162 A radiation dose of more than 80 Gy causes partial degeneration with loss of axons and demyelination, which is the mechanism of pain relief in GKRS.62 Therefore, GKRS with 80 Gy or higher is usually performed. In addition, many studies have shown that higher doses lead to greater pain control.636465 However, a high dose of more than 90 Gy is related to a higher complication rate with similar pain control effects; hence, they are not usually used.56 Recently, a dose between 80 and 90 Gy is generally used, with modification depending the circumstances.
GKRS has been used for TN for a long time with low complication rates and high success rates. Over time, technical refinements have improved its safety and efficacy. GKRS is being increasingly used as a primary intervention for TN for patients who cannot undergo surgery due to medical comorbidities and age or for those who refuse invasive therapy. To further increase safety and efficiency, discussions are underway on the treatment policy to be applied. In the future, advances in imaging modalities and in GKRS technique, as well as accumulation of long-term results and experience will yield better results of GRKS for TN.
Notes
AUTHOR CONTRIBUTIONS:
Conceptualization: Jin Woo Chang.
Data curation: So Hee Park.
Formal analysis: So Hee Park.
Methodology: Jin Woo Chang and So Hee Park.
Project administration: Jin Woo Chang.
Resources: Jin Woo Chang and So Hee Park.
Software: So Hee Park.
Supervision: Jin Woo Chang.
Validation: Jin Woo Chang.
Visualization: So Hee Park.
Writing—original draft: So Hee Park.
Writing—review & editing: Jin Woo Chang and So Hee Park.
Approval of final manuscript: all authors.
References
1. Headache Classification Committee of the International Headache Society (IHS). The international classification of headache disorders, 3rd edition (beta version). Cephalalgia. 2013; 33:629–808. PMID: 23771276.
3. Leksell L. The stereotactic method and radiosurgery of the brain. Acta Chir Scand. 1951; 102:316–319. PMID: 14914373.
4. Lindquist C, Kihlström L, Hellstrand E. Functional neurosurgery--a future for the gamma knife? Stereotact Funct Neurosurg. 1991; 57:72–81. PMID: 1725560.
5. Kondziolka D, Zorro O, Lobato-Polo J, Kano H, Flannery TJ, Flickinger JC, et al. Gamma Knife stereotactic radiosurgery for idiopathic trigeminal neuralgia. J Neurosurg. 2010; 112:758–765. PMID: 19747055.

6. Régis J, Tuleasca C, Resseguier N, Carron R, Donnet A, Gaudart J, et al. Long-term safety and efficacy of Gamma Knife surgery in classical trigeminal neuralgia: a 497-patient historical cohort study. J Neurosurg. 2016; 124:1079–1087. PMID: 26339857.

7. Romanelli P, Heit G, Chang SD, Martin D, Pham C, Adler J. Cyberknife radiosurgery for trigeminal neuralgia. Stereotact Funct Neurosurg. 2003; 81:105–109. PMID: 14742972.

8. Smith ZA, Gorgulho AA, Bezrukiy N, McArthur D, Agazaryan N, Selch MT, et al. Dedicated linear accelerator radiosurgery for trigeminal neuralgia: a single-center experience in 179 patients with varied dose prescriptions and treatment plans. Int J Radiat Oncol Biol Phys. 2011; 81:225–231. PMID: 21236592.

9. Costa GMF, Leite CMdA. Trigeminal neuralgia: peripheral and central mechanisms. Revista Dor. 2015; 16:297–301.

10. Yadav YR, Nishtha Y, Sonjjay P, Vijay P, Shailendra R, Yatin K. Trigeminal neuralgia. Asian J Neurosurg. 2017; 12:585–597. PMID: 29114270.

11. Kumar S, Rastogi S, Kumar S, Mahendra P, Bansal M, Chandra L. Pain in trigeminal neuralgia: neurophysiology and measurement: a comprehensive review. J Med Life. 2013; 6:383–388. PMID: 24701256.
12. Love S, Coakham HB. Trigeminal neuralgia: pathology and pathogenesis. Brain. 2001; 124:2347–2360. PMID: 11701590.

13. Marinković S, Gibo H, Todorović V, Antić B, Kovacević D, Milisavljević M, et al. Ultrastructure and immunohistochemistry of the trigeminal peripheral myelinated axons in patients with neuralgia. Clin Neurol Neurosurg. 2009; 111:795–800. PMID: 19836877.

14. Burchiel KJ. A new classification for facial pain. Neurosurgery. 2003; 53:1164–1166. PMID: 14580284.

15. Wiffen PJ, McQuay HJ, Moore RA. Carbamazepine for acute and chronic pain. Cochrane Database Syst Rev. 2005; CD005451. PMID: 16034977.
16. Montano N, Conforti G, Di Bonaventura R, Meglio M, Fernandez E, Papacci F. Advances in diagnosis and treatment of trigeminal neuralgia. Ther Clin Risk Manag. 2015; 11:289–299. PMID: 25750533.
17. Cruccu G, Gronseth G, Alksne J, Argoff C, Brainin M, Burchiel K, et al. AAN-EFNS guidelines on trigeminal neuralgia management. Eur J Neurol. 2008; 15:1013–1028. PMID: 18721143.

18. Barker FG, Jannetta PJ, Bissonette DJ, Larkins MV, Jho HD. The long-term outcome of microvascular decompression for trigeminal neuralgia. N Engl J Med. 1996; 334:1077–1084. PMID: 8598865.

19. Burchiel KJ, Clarke H, Haglund M, Loeser JD. Long-term efficacy of microvascular decompression in trigeminal neuralgia. J Neurosurg. 1988; 69:35–38. PMID: 2454303.

20. Sekula RF Jr, Frederickson AM, Jannetta PJ, Quigley MR, Aziz KM, Arnone GD. Microvascular decompression for elderly patients with trigeminal neuralgia: a prospective study and systematic review with meta-analysis. J Neurosurg. 2011; 114:172–179. PMID: 20653393.

21. Yang DB, Wang ZM, Jiang DY, Chen HC. The efficacy and safety of microvascular decompression for idiopathic trigeminal neuralgia in patients older than 65 years. J Craniofac Surg. 2014; 25:1393–1396. PMID: 24816027.

22. Parmar M, Sharma N, Modgill V, Naidu P. Comparative evaluation of surgical procedures for trigeminal neuralgia. J Maxillofac Oral Surg. 2013; 12:400–409. PMID: 24431878.

23. Park SS, Lee MK, Kim JW, Jung JY, Kim IS, Ghang CG. Percutaneous balloon compression of trigeminal ganglion for the treatment of idiopathic trigeminal neuralgia: experience in 50 patients. J Korean Neurosurg Soc. 2008; 43:186–189. PMID: 19096641.
24. Asplund P, Blomstedt P, Bergenheim AT. Percutaneous balloon compression vs percutaneous retrogasserian glycerol rhizotomy for the primary treatment of trigeminal neuralgia. Neurosurgery. 2016; 78:421–428. PMID: 26465639.

25. Pollock BE. Percutaneous retrogasserian glycerol rhizotomy for patients with idiopathic trigeminal neuralgia: a prospective analysis of factors related to pain relief. J Neurosurg. 2005; 102:223–228. PMID: 15739548.

26. Yoon KB, Wiles JR, Miles JB, Nurmikko TJ. Long-term outcome of percutaneous thermocoagulation for trigeminal neuralgia. Anaesthesia. 1999; 54:803–808. PMID: 10460537.

27. Wu CY, Meng FG, Xu SJ, Liu YG, Wang HW. Selective percutaneous radiofrequency thermocoagulation in the treatment of trigeminal neuralgia: report on 1860 cases. Chin Med J (Engl). 2004; 117:467–470. PMID: 15043796.
28. Fouad W. Management of trigeminal neuralgia by radiofrequency thermocoagulation. Alex J Med. 2011; 47:79–86.

29. Tuleasca C, Régis J, Sahgal A, De Salles A, Hayashi M, Ma L, et al. Stereotactic radiosurgery for trigeminal neuralgia: a systematic review: International Stereotactic Radiosurgery Society Practice Guidelines. J Neurosurg. 2018; 130:733–757. PMID: 29701555.
30. Tang YZ, Wu BS, Yang LQ, Yue JN, He LL, Li N, et al. The longterm effective rate of different branches of idiopathic trigeminal neuralgia after single radiofrequency thermocoagulation: a cohort study. Medicine (Baltimore). 2015; 94:e1994. PMID: 26559288.
31. Keep MF, DeMare PA, Ashby LS. Gamma knife surgery for refractory postherpetic trigeminal neuralgia: targeting in one session both the retrogasserian trigeminal nerve and the centromedian nucleus of the thalamus. J Neurosurg. 2005; 102:276–282.

32. Tuleasca C, Carron R, Resseguier N, Donnet A, Roussel P, Gaudart J, et al. Multiple sclerosis-related trigeminal neuralgia: a prospective series of 43 patients treated with gamma knife surgery with more than one year of follow-up. Stereotact Funct Neurosurg. 2014; 92:203–210. PMID: 25011487.

33. Schwarz W, Fox JM. Effects of monochromatic X-radiation on the membrane of nodes of Ranvier under voltage and current clamp conditions. Experientia. 1979; 35:1200–1201. PMID: 314907.

34. Szeifert GT, Salmon I, Lorenzoni J, Massager N, Levivier M. Pathological findings following trigeminal neuralgia radiosurgery. Prog Neurol Surg. 2007; 20:244–248. PMID: 17317993.

35. Maesawa S, Salame C, Flickinger JC, Pirris S, Kondziolka D, Lunsford LD. Clinical outcomes after stereotactic radiosurgery for idiopathic trigeminal neuralgia. J Neurosurg. 2001; 94:14–20. PMID: 11147887.

36. Gronseth G, Cruccu G, Alksne J, Argoff C, Brainin M, Burchiel K, et al. Practice parameter: the diagnostic evaluation and treatment of trigeminal neuralgia (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology and the European Federation of Neurological Societies. Neurology. 2008; 71:1183–1190. PMID: 18716236.

37. Little AS, Shetter AG, Shetter ME, Bay C, Rogers CL. Long-term pain response and quality of life in patients with typical trigeminal neuralgia treated with gamma knife stereotactic radiosurgery. Neurosurgery. 2008; 63:915–924. PMID: 19005382.

38. Urgosik D, Liscak R, Novotny J Jr, Vymazal J, Vladyka V. Treatment of essential trigeminal neuralgia with gamma knife surgery. J Neurosurg. 2005; 102:29–33.

39. Rogers CL, Shetter AG, Fiedler JA, Smith KA, Han PP, Speiser BL. Gamma knife radiosurgery for trigeminal neuralgia: the initial experience of The Barrow Neurological Institute. Int J Radiat Oncol Biol Phys. 2000; 47:1013–1019. PMID: 10863073.

40. Longhi M, Rizzo P, Nicolato A, Foroni R, Reggio M, Gerosa M. Gamma knife radiosurgery for trigeminal neuralgia: results and potentially predictive parameters--part I: idiopathic trigeminal neuralgia. Neurosurgery. 2007; 61:1254–1261. PMID: 18162905.
41. Guo S, Chao ST, Reuther AM, Barnett GH, Suh JH. Review of the treatment of trigeminal neuralgia with gamma knife radiosurgery. Stereotact Funct Neurosurg. 2008; 86:135–146. PMID: 18334855.

42. Pollock BE, Phuong LK, Gorman DA, Foote RL, Stafford SL. Stereotactic radiosurgery for idiopathic trigeminal neuralgia. J Neurosurg. 2002; 97:347–353. PMID: 12186463.

43. Maher CO, Pollock BE. Radiation induced vascular injury after stereotactic radiosurgery for trigeminal neuralgia: case report. Surg Neurol. 2000; 54:189–193. PMID: 11077103.

44. Marshall K, Chan MD, McCoy TP, Aubuchon AC, Bourland JD, McMullen KP, et al. Predictive variables for the successful treatment of trigeminal neuralgia with gamma knife radiosurgery. Neurosurgery. 2012; 70:566–573. PMID: 21849918.

45. Tang X, Wu H, Wang B, Zhang N, Dong Y, Ding J, et al. A new classification and clinical results of Gamma Knife radiosurgery for cavernous sinus hemangiomas: a report of 53 cases. Acta Neurochir (Wien). 2015; 157:961–969. PMID: 25862173.

46. McNatt SA, Yu C, Giannotta SL, Zee C-S, Zelman V, Apuzzo ML, et al. Gamma knife radiosurgery for trigeminal neuralgia. Neurosurgery. 2005; 56:1295–1303. PMID: 15918946.

47. Young B, Shivazad A, Kryscio RJ, St Clair W, Bush HM. Long-term outcome of high-dose γ knife surgery in treatment of trigeminal neuralgia. J Neurosurg. 2013; 119:1166–1175. PMID: 23600932.
48. Fountas KN, Smith JR, Lee GP, Jenkins PD, Cantrell RR, Sheils WC. Gamma Knife stereotactic radiosurgical treatment of idiopathic trigeminal neuralgia: long-term outcome and complications. Neurosurg Focus. 2007; 23:E8.

49. Taich ZJ, Goetsch SJ, Monaco E, Carter BS, Ott K, Alksne JF, et al. Stereotactic radiosurgery treatment of trigeminal neuralgia: clinical outcomes and prognostic factors. World Neurosurg. 2016; 90:604–612.e11. PMID: 26915701.

50. Zhao H, Shen Y, Yao D, Xiong N, Abdelmaksoud A, Wang H. Outcomes of two-isocenter gamma knife radiosurgery for patients with typical trigeminal neuralgia: pain response and quality of life. World Neurosurg. 2018; 109:e531–e538. PMID: 29038085.

51. Nicol B, Regine WF, Courtney C, Meigooni A, Sanders M, Young B. Gamma knife radiosurgery using 90 Gy for trigeminal neuralgia. J Neurosurg. 2000; 93:152–154.

52. Massager N, Nissim O, Murata N, Devriendt D, Desmedt F, Vanderlinden B, et al. Effect of beam channel plugging on the outcome of gamma knife radiosurgery for trigeminal neuralgia. Int J Radiat Oncol Biol Phys. 2006; 65:1200–1205. PMID: 16682146.

53. Pollock BE, Foote RL, Stafford SL, Link MJ, Gorman DA, Schomberg PJ. Results of repeated gamma knife radiosurgery for medically unresponsive trigeminal neuralgia. J Neurosurg. 2000; 93:162–164.

54. Hasegawa T, Kondziolka D, Spiro R, Flickinger JC, Lunsford LD. Repeat radiosurgery for refractory trigeminal neuralgia. Neurosurgery. 2002; 50:494–500. PMID: 11841716.

55. Pollock BE, Foote RL, Link MJ, Stafford SL, Brown PD, Schomberg PJ. Repeat radiosurgery for idiopathic trigeminal neuralgia. Int J Radiat Oncol Biol Phys. 2005; 61:192–195. PMID: 15629611.

56. Aubuchon AC, Chan MD, Lovato JF, Balamucki CJ, Ellis TL, Tatter SB, et al. Repeat gamma knife radiosurgery for trigeminal neuralgia. Int J Radiat Oncol Biol Phys. 2011; 81:1059–1065. PMID: 20932665.

57. Rand RW, Jacques DB, Melbye RW, Copcutt BG, Levenick MN, Fisher MR. Leksell Gamma Knife treatment of tic douloureux. Stereotact Funct Neurosurg. 1993; 61:93–102. PMID: 8115760.

58. Xu Z, Schlesinger D, Moldovan K, Przybylowski C, Sun X, Lee CC, et al. Impact of target location on the response of trigeminal neuralgia to stereotactic radiosurgery. J Neurosurg. 2014; 120:716–724. PMID: 24313616.

59. Matsuda S, Serizawa T, Nagano O, Ono J. Comparison of the results of 2 targeting methods in Gamma Knife surgery for trigeminal neuralgia. J Neurosurg. 2008; 109:185–189. PMID: 19123907.

60. Park SH, Hwang SK, Kang DH, Park J, Hwang JH, Sung JK. The retrogasserian zone versus dorsal root entry zone: comparison of two targeting techniques of gamma knife radiosurgery for trigeminal neuralgia. Acta Neurochir (Wien). 2010; 152:1165–1170. PMID: 20204664.

61. Shaya M, Jawahar A, Caldito G, Sin A, Willis BK, Nanda A. Gamma knife radiosurgery for trigeminal neuralgia: a study of predictors of success, efficacy, safety, and outcome at LSUHSC. Surg Neurol. 2004; 61:529–534. PMID: 15165787.

62. Zhao ZF, Yang LZ, Jiang CL, Zheng YR, Zhang JW. Gamma Knife irradiation-induced histopathological changes in the trigeminal nerves of rhesus monkeys. J Neurosurg. 2010; 113:39–44.

63. Shrivastava A, Mohammed N, Hung YC, Xu Z, Schlesinger D, Heinrichs T, et al. Impact of integral dose on the maintenance of pain relief in patients with idiopathic trigeminal neuralgia treated with upfront Gamma knife radiosurgery. World Neurosurg. 2019; 129:e375–e380. PMID: 31132503.

64. Morbidini-Gaffney S, Chung CT, Alpert TE, Newman N, Hahn SS, Shah H, et al. Doses greater than 85 Gy and two isocenters in Gamma Knife surgery for trigeminal neuralgia: updated results. J Neurosurg. 2006; 105:107–111.

65. Kim YH, Kim DG, Kim JW, Kim YH, Han JH, Chung HT, et al. Is it effective to raise the irradiation dose from 80 to 85 Gy in gamma knife radiosurgery for trigeminal neuralgia? Stereotact Funct Neurosurg. 2010; 88:169–176. PMID: 20431328.

66. Young RF, Vermulen S, Posewitz A. Gamma knife radiosurgery for the treatment of trigeminal neuralgia. Stereotact Funct Neurosurg. 1998; 70 Suppl 1:192–199. PMID: 9782251.

67. Petit JH, Herman JM, Nagda S, DiBiase SJ, Chin LS. Radiosurgical treatment of trigeminal neuralgia: evaluating quality of life and treatment outcomes. Int J Radiat Oncol Biol Phys. 2003; 56:1147–1153. PMID: 12829153.

68. Sheehan J, Pan HC, Stroila M, Steiner L. Gamma knife surgery for trigeminal neuralgia: outcomes and prognostic factors. J Neurosurg. 2005; 102:434–441. PMID: 15796376.

69. Hayashi M, Chernov M, Tamura N, Taira T, Izawa M, Yomo S, et al. Stereotactic radiosurgery of essential trigeminal neuralgia using Leksell Gamma Knife model C with automatic positioning system: technical nuances and evaluation of outcome in 130 patients with at least 2 years follow-up after treatment. Neurosurg Rev. 2011; 34:497. PMID: 21701866.
70. Lucas JT Jr, Nida AM, Isom S, Marshall K, Bourland JD, Laxton AW, et al. Predictive nomogram for the durability of pain relief from gamma knife radiation surgery in the treatment of trigeminal neuralgia. Int J Radiat Oncol Biol Phys. 2014; 89:120–126. PMID: 24613811.

71. Martínez Moreno NE, Gutiérrez-Sárraga J, Rey-Portolés G, Jiménez-Huete A, Martínez Álvarez R. Long-term outcomes in the treatment of classical trigeminal neuralgia by Gamma Knife radiosurgery: a retrospective study in patients with minimum 2-year follow-up. Neurosurgery. 2016; 79:879–888. PMID: 27560193.
72. Gagliardi F, Spina A, Bailo M, Boari N, Cavalli A, Franzin A, et al. Effectiveness of Gamma Knife Radiosurgery in improving psychophysical performance and patient's quality of life in idiopathic trigeminal neuralgia. World Neurosurg. 2018; 110:e776–e785. PMID: 29174233.

73. Lee CC, Chen CJ, Chong ST, Hung SC, Yang HC, Lin CJ, et al. Early stereotactic radiosurgery for medically refractory trigeminal neuralgia. World Neurosurg. 2018; 112:e569–e575. PMID: 29371169.

Fig. 1
Flow chart for patient inclusion. TN, trigeminal neuralgia; GKRS, Gamma Knife radiosurgery; MS, multiple sclerosis.
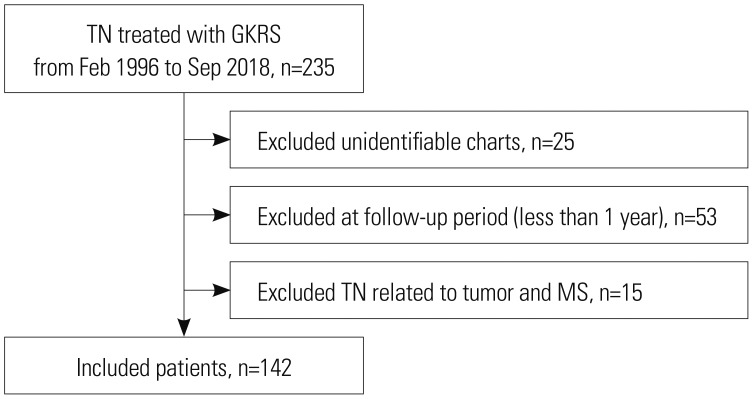
Fig. 2
Predicted pain relief maintenance period after Gamma Knife radiosurgery. Solid line represents predicted pain relief maintenance period and dotted line represents 95% confidence interval.
Fig. 3
Flow chart for inclusion of studies analyzing pain relief, complication, and recurrence rates.
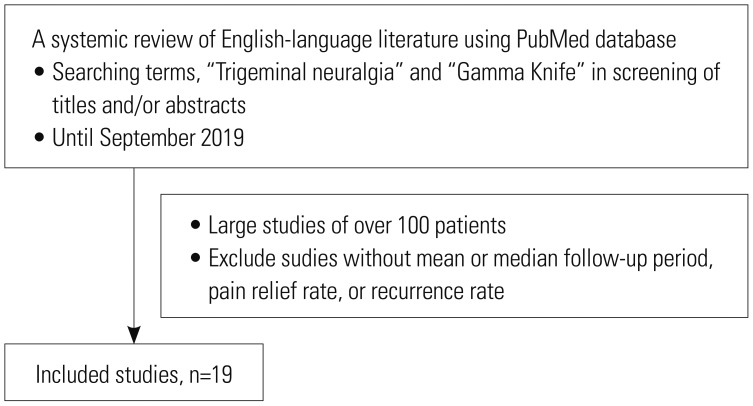
Fig. 4
Forest plot of pain relief rates in 19 studies. Confidence intervals were calculated at a confidence level of 95% for a single proportion.
Fig. 5
Forest plot of complication rates in 19 studies. Confidence intervals were calculated at a confidence level of 95% for a single proportion.
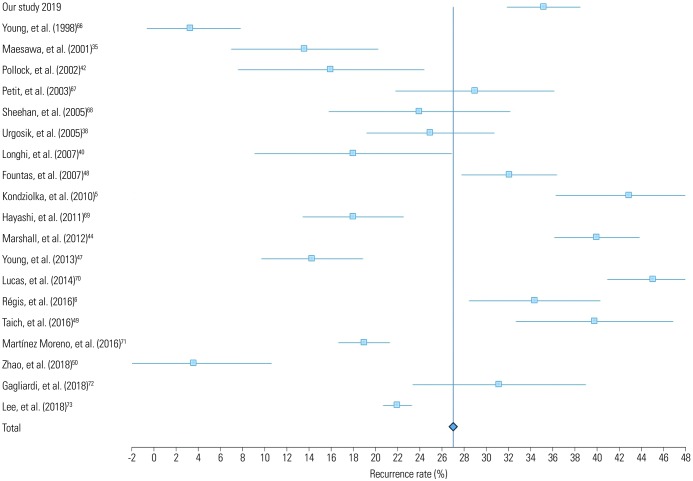
Fig. 6
Targets used in Gamma Knife radiosurgery for trigeminal neuralgia. Retrogasserian area (A) and trigeminal root entry zone (B).
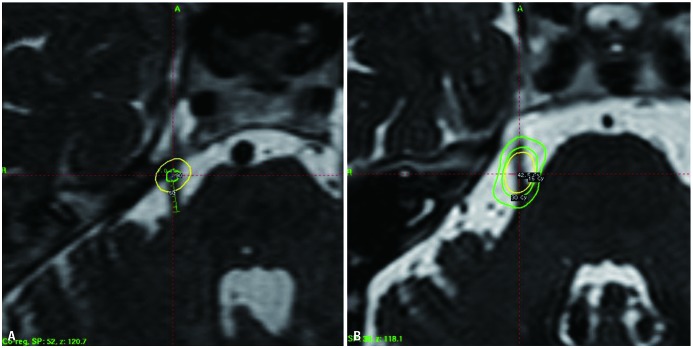
Table 1
Burchiel Classification of TN and Related Facial Pain Syndromes
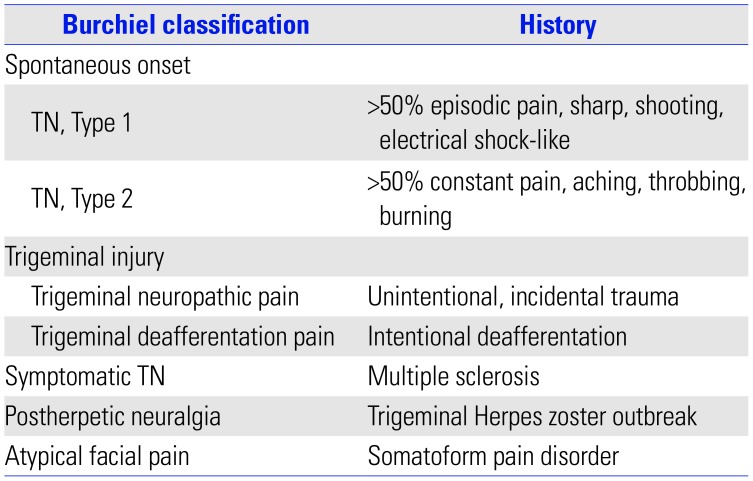
Table 2
Clinical Characteristics of Patients
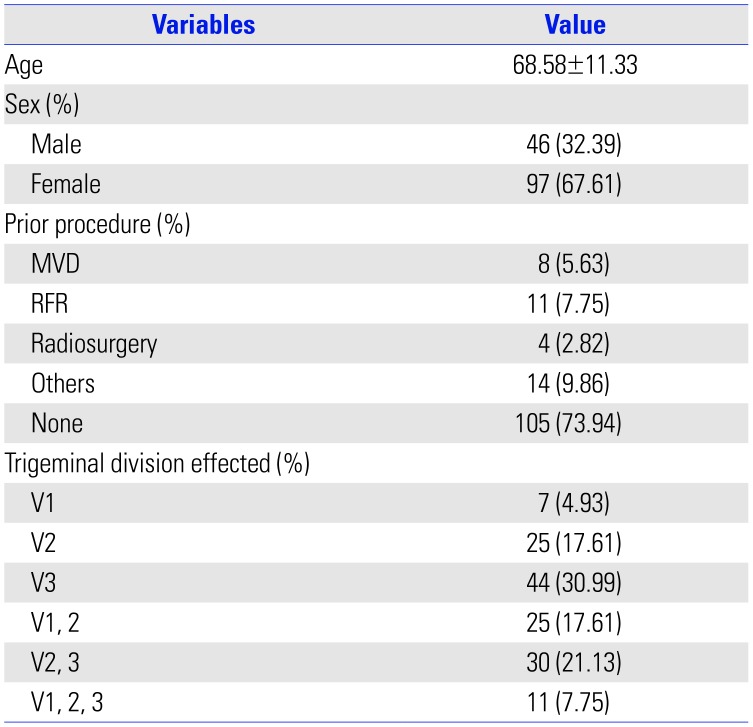
Table 3
Results of Gamma Knife Radiosurgery for Trigeminal Neuralgia
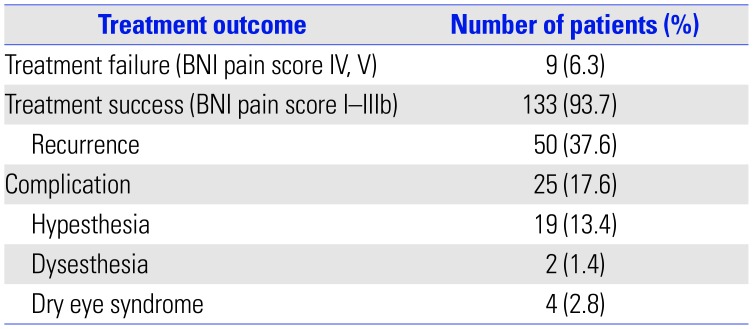
Table 4
Review of Studies with more than 100 Cases of Gamma Knife Radiosurgery for Trigeminal Neuralgia
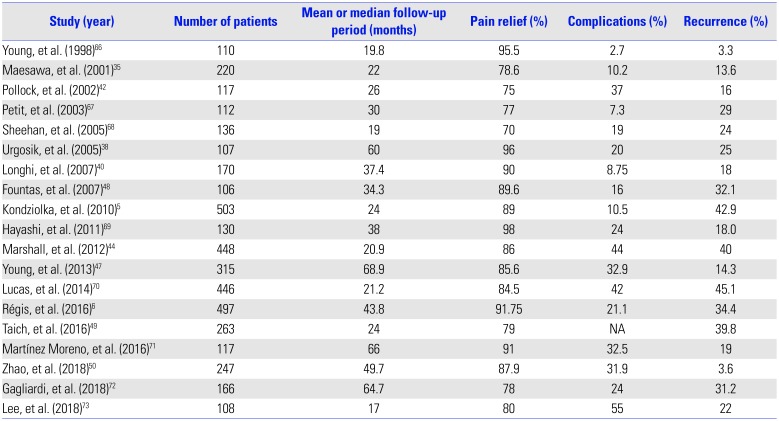
| Study (year) | Number of patients | Mean or median follow-up period (months) | Pain relief (%) | Complications (%) | Recurrence (%) |
|---|---|---|---|---|---|
| Young, et al. (1998)66 | 110 | 19.8 | 95.5 | 2.7 | 3.3 |
| Maesawa, et al. (2001)35 | 220 | 22 | 78.6 | 10.2 | 13.6 |
| Pollock, et al. (2002)42 | 117 | 26 | 75 | 37 | 16 |
| Petit, et al. (2003)67 | 112 | 30 | 77 | 7.3 | 29 |
| Sheehan, et al. (2005)68 | 136 | 19 | 70 | 19 | 24 |
| Urgosik, et al. (2005)38 | 107 | 60 | 96 | 20 | 25 |
| Longhi, et al. (2007)40 | 170 | 37.4 | 90 | 8.75 | 18 |
| Fountas, et al. (2007)48 | 106 | 34.3 | 89.6 | 16 | 32.1 |
| Kondziolka, et al. (2010)5 | 503 | 24 | 89 | 10.5 | 42.9 |
| Hayashi, et al. (2011)69 | 130 | 38 | 98 | 24 | 18 |
| Marshall, et al. (2012)44 | 448 | 20.9 | 86 | 44 | 40 |
| Young, et al. (2013)47 | 315 | 68.9 | 85.6 | 32.9 | 14.3 |
| Lucas, et al. (2014)70 | 446 | 21.2 | 84.5 | 42 | 45.1 |
| Régis, et al. (2016)6 | 497 | 43.8 | 91.75 | 21.1 | 34.4 |
| Taich, et al. (2016)49 | 263 | 24 | 79 | NA | 39.8 |
| Martínez Moreno, et al. (2016)71 | 117 | 66 | 91 | 32.5 | 19 |
| Zhao, et al. (2018)50 | 247 | 49.7 | 87.9 | 31.9 | 3.6 |
| Gagliardi, et al. (2018)72 | 166 | 64.7 | 78 | 24 | 31.2 |
| Lee, et al. (2018)73 | 108 | 17 | 80 | 55 | 22 |




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download