INTRODUCTION
 | Fig. 1Cross-cutting initiatives of 2017-2019 EIP on AHA.EIP on AHA, European Innovation Partnership on Active and Healthy Ageing; MAFEIP, monitoring and assessment framework.
|
THE MOBILE HEALTH (mHEALTH) GOOD PRACTICE MASK
MASK-air®
Table
MASK air®

| • GP of the EIP on AHA follows CHRODIS59 |
| • GP on digitally enabled, integrated, patient-centred care endorsed by DG Santé4 |
| • Based on several EU grants (MeDALL, GA2LEN56) including—in 2018—POLLAR,21 DHE Twinning (digital transformation of health). |
| • Reported in the JRC Science and Policy Reports on Strategic Intelligence Monitor on Personal Health Systems phase 37 |
| • One example of the WHO-ITU “Be He@lthy, Be Mobile” handbook on how to implement mBreatheFreely for asthma and COPD50 |
| • GARD (WHO alliance) demonstration project |
• App: 30,000 users, 23 countries, 17 languages
• 250,000 days of VAS report
• No missing data due to app structure
• Tested with patients and physicians for acceptability
• GDPR including geolocation
• Follows the recommendations of ICPs for airway diseases (AIRWAYS ICPs)15
• From a validated “research” tool (2004-2018) to large scale deployment (2019-)
• Validation with COnsensus-based Standards for the selection of health Measurement INstruments guidelines20
• Found to be the most relevant app for rhinitis and asthma2122
• Assessment of data quality (Bedard, in preparation)
• Baseline characteristics23
• Work productivity16
• EuroQOL (EQ-5D) and the work productivity and activity impairment allergy-specific7
• Novel phenotypes of allergic diseases24
• Novel approaches to inform the efficacy of treatment25
• Sleep23
• Patient's organizations and scientific societies involved
• Presented during WHO, EU ministerial meetings and EU parliament meetings12272829
• Next-generation care pathways meeting (December 3, 2018) with the EIP on AHA, POLLAR and GARD
• Over 70 MASK papers in 16 languages
• Dissemination according to the EIP on AHA10
Web-based physician's questionnaire for rhinitis and asthma
MANAGEMENT OF CARE PROCESSES
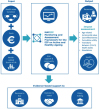
BLUEPRINT OF DIGITAL TRANSFORMATION
Data analytics for predictive risk stratification and prevention
Pro-active prevention through empowerment, self-management, monitoring and coaching
Regions with positive experiences willing to provide the necessary knowledge and support to scale up and deploy across Europe
Relevant interactions
Need of other key actors
High scalability and replication potential
EIP ON AHA, I2M
Visibility and awareness activities
Adoption award: MASK has already proposed 2 types of awards
• An award (platinum, gold or silver) for the members who have had the most important activities in the network (Campania, Piedimonte, Puglia, Lithuania, Mexico and Brazil received the platinum award).
• ARIACare on the model of Ucare (for urticaria),43 in collaboration with the Global Allergy and Asthma European Network, GA2LEN.44 GA2LEN has launched a program for the development, interaction and accreditation of center of reference and excellence in special areas of allergy embedded in its overall quality management of allergy center of excellence. The first chosen area is urticaria. From September 2019, ARIACare will accredit and award the center in AR and asthma based on the ARIA study group of 600 members. The call preparation and the jury are in place. It is expected that 100 centers will be awarded globally.
Expanding repository of innovative practices
Needs showcase and solution platforms
Knowledge brokerage and matchmaking services
Transfer activities (Twinning schemes)
Dissemination of MASK
COMMUNITY FOR MAFEIP
POLITICAL, ORGANIZATIONAL, TECHNOLOGICAL AND FINANCIAL READINESS
Political
Regional
• MASK is fully supported by the Région Occitanie.
• In a project on transfer of innovation for severe asthma, the engagement through Salerno local health agency of ProMIS@Campania network4849 will ensure that adoption is progressively achieved through a multicentric scale-up pilot. The GP will be adopted by Campania Reference Site through Salerno Health Agency. The involvement of Campania Region Health Directorate through the Unit for Health Innovation will ensure progressive further scale-up to the regional health system through the ProMIS@Campania network. The National ProMIS network will support dissemination and exploitation to other Italian regions.
Political agenda of the EU
• The EU political agenda is of great importance in supporting the digital transformation of health and care for CRDs. The Polish Presidency of the EU Council (2011) prioritized the early diagnosis, prevention and control of CRDs in children.5152 AIRWAYS-ICPs,53 initiated in 2014 by the EIP on AHA,54 launched a collaboration to develop multi-sectoral ICPs. It was a GARD demonstration project.11
• Euforea proposed a yearly stepwise strategy at the EU or ministerial levels.55 Euforea organized an EU Summit in Vilnius, Lithuania (March 2018) to propose multisectoral ICPs embedding guided self-management, mHealth and air pollution in CRDs.15 On May 3, 2019, another Euforea-led meeting (Parliament of Malta) reviewed the results of the Vilnius Declaration.
• POLLAR is focusing on the impact of allergens and air pollution on airway diseases to propose novel ICPs integrating pollution, sleep and patient literacy.15
• The next phase of MASK will be the digital transformation of health and care to sustain Planetary health.
Organizational
• GP of the EIP on AHA follows CHRODIS.56
• GP on digitally enabled, integrated, patient-centered care endorsed by DG Santé 3
• Based on several EU grants (Mechanisms of the Development of ALLergy,57 GA2LEN44) including POLLAR,15 and the DHE TWINNING on the Digital Single Market.
• Reported in the Joint Research Centre Science and Policy Reports on Strategic Intelligence Monitor on Personal Health Systems phase 3.58
Technology Readiness level (TRL)
• App (MASK-air®): TRL9 (ISO 16290:2013 standard59).
• e-physician questionnaire deployed in 23 countries (available on the Euforea website): TRL9.
• Electronic clinical decision support system for the tablet (ARIA e-CDSS): TRL7.
• App (MASK-asthma): TRL8 and tested in 23 countries 4-2020 (TRL9).
• Embedding air pollution and pollen data in MASK-air® (POLLAR): TRL9.
• Embedding artificial intelligence in MASK-air®: TLR3.
Financial readiness
CONTRIBUTION TO EUROPEAN CO-OPERATION AND TRANSFERABILITY
Rhinitis and asthma Twinning (2017-2018)
GARD: target on developing countries
Euforea
DELIVERING EVIDENCE OF IMPACT AGAINST THE TRIPLE WIN APPROACH
Quality in care (individual benefit)
• MASK ensured that mHealth apps meet citizens' demands for quality and transparency.
• MASK was tested for quality and compared favorably with other mHealth apps for CRDs.
• MASK adheres to strict data protection rules. MASK follows GDPR strictly in particular for geolocation using the k-anonymity method.
• MASK attempts to increase user trust and patient safety in order to boost mHealth's contribution to high-quality healthcare.
• MASK attempts to use novel methods about how we use the data. MASK provided real-world information on rhinitis and asthma.
Research and innovation (industrial benefit)
• MASK has immediately involved SMEs (Kyomed and ASA, Montpellier, and Peercode, NL) to develop the project with a strong business plan for the GP Solution. A new start-up has been established (MASK-air) initially creating 4 job opportunities. MASK-air will develop centers of excellence on digital health for airway diseases (ARIACare-Digital).
• MASK obtained an EIT Health project (POLLAR) to develop an innovative solution in order to predict the pollen season and its interactions with pollution using a business plan and a strong commercial commitment.
Supporting the long-term sustainability and efficiency of health and social care systems (institutional and staff benefit)
CONTRIBUTION TO THE EUROPEAN DIGITAL TRANSFORMATION OF HEALTH AND CARE (MASK─A GP OF DG SANTÉ)
1) Patient participation, health literacy and self-care through technology-assisted “patient activation.”29
2) ICP implementation by pharmacists.37
3) Next-generation ARIA guidelines have assessed the GRADE recommendations in AR and asthma using RWE that includes not only randomized controlled trials on treatment effects, but also evidence obtained by mHealth tools including MASK in order to confirm the efficiency or refine current recommendations. The MASK results have confirmed the feasibility of the project that will be used for other diseases.
4) ARIA ICPs for allergen immunotherapy,67 including an innovative symptom-medication score, based on the real-world data of MASK and the results of POLLAR for the prediction of the pollen season and pollutants.
5) Embedding air pollution, aerobiology and novel approaches in ICPs during a meeting organized by the Finnish Institute of Health and Welfare (Presidency of the EU council, December 3-4, 2019).




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download