Abstract
We examined the effect of fluoxetine, a selective serotonin reuptake inhibitor antidepressant, on neuronal viability in mouse cortical near-pure neuronal cultures. Addition of fluoxetine to the media for 24 hours induced neuronal death in a concentration-dependent manner. To delineate the mechanisms of fluoxetine-induced neuronal death, we investigated the effects of trolox, cycloheximide (CHX), BDNF, z-VAD-FMK, and various metal-chelators on fluoxetine-induced neuronal death. Neuronal death was assessed by MTT assay. The addition of 20 µM fluoxetine to the media for 24 hours induced 60–70% neuronal death, which was associated with the hallmarks of apoptosis, chromatin condensation and DNA laddering. Fluoxetine-induced death was significantly attenuated by CHX, BDNF, or z-VAD-FMK. Treatment with antioxidants, trolox and ascorbate, also markedly attenuated fluoxetine-induced death. Interestingly, some divalent cation chelators (EGTA, Ca-EDTA, and Zn-EDTA) also markedly attenuated the neurotoxicity. Fluoxetine-induced reactive oxygen species (ROS) generation was measured using the fluorescent dye 2′,7′-dichlorofluorescin diacetate. Trolox and bathocuproine disulfonic acid (BCPS), a cell membrane impermeable copper ion chelator, markedly attenuated the ROS production and neuronal death. However, deferoxamine, an iron chelator, did not affect ROS generation or neurotoxicity. We examined the changes in intracellular copper concentration using a copper-selective fluorescent dye, Phen Green FL, which is quenched by free copper ions. Fluoxetine quenched the fluorescence in neuronal cells, and the quenching effect of fluoxetine was reversed by co-treatment with BCPS, however, not by deferoxamine. These findings demonstrate that fluoxetine could induce apoptotic and oxidative neuronal death associated with an influx of copper ions.
Fluoxetine is a well-known selective serotonin reuptake inhibitor (SSRI) antidepressant.1 Besides its inhibitory action on the serotonin transporter, fluoxetine has many effects on several ion channels such as inhibition of the voltage-dependent Na+, K+, and Ca2+ channels;234 modification of mitochondrial voltage-dependent anion channel activity;5 blockade of the nicotinic receptor;6 and inhibition of multidrug resistance pumps7 and the NMDA receptor.8
Fluoxetine has been reported to potentially induce cell proliferation and increase cancer risk.9 However, growing evidence suggests that fluoxetine induces death of various tumor cells such as C6 glioma cells10 and those found in colon cancer,11 Burkitt lymphoma,12 and glioblastoma.1314 Recently, some anti-psychotic drugs, including fluoxetine, have become drug-repositioning candidates for glioblastoma multiforme.15
Since the neurotrophic hypothesis of depression was suggested,16 it has been reported that antidepressants, especially SSRIs, increase adult neurogenesis,1718 expression of neurotrophic factors19 and synaptogenesis,20 along with having neuroprotective actions.21 In contrast, antidepressants, including fluoxetine, induce apoptosis in neuronal cells.2223
We examined the effect of fluoxetine on neuronal survival in murine cortical near-pure neuronal cultures and found that fluoxetine unexpectedly induced neuronal death.
In this study, we investigated the effects of various antioxidants, anti-apoptotic agents, and metal-chelators to delineate the mechanisms of fluoxetine-induced neuronal death. We demonstrate that fluoxetine induces apoptotic and oxidative neuronal death associated with an influx of copper ions in mouse neuronal cultures.
Primary mouse cortical near-pure neuronal cultures containing less than 1% astrocytes were prepared as previously described.24 Fetal mice were rapidly removed from euthanized pregnant (14–15 days gestation) ICR mice (Institute of Cancer Research, Damool, Korea) and decapitated. Brains were excised and then rinsed in cold Ca2+/Mg2+-free Hank's balanced salt solution supplemented with 5 mg/mL glucose, 7 mg/mL sucrose, and 0.35 mg/mL sodium bicarbonate (DM). Using fine-tipped forceps and a microsurgical knife, the meninges was carefully removed from the brain tissue under a stereomicroscope. The cerebral cortex was removed and cut into 1-2 mm3 sized pieces with a sterile scalpel. The cortex pieces were centrifuged at 1,000×g for 5 minutes. After removal of supernatant, the tissue pellet was suspended in 1–2 mL plating medium with Eagle's minimal essential medium (MEM; Gibco, Gaithersburg, MD, USA) containing 2 mM glutamine, 5% fetal bovine serum, and 5% horse serum. Cells were separated by 8 or 10 trituration passages using a flame-narrowed pipette. Dissociated cortical cells were plated onto polyethyleneimine-coated 24-well plates at a density of 4-5 hemispheres/plate. The plates were placed in a Forma incubator (Fisher Scientific, Hampton, NH, USA), at 37℃, 5% CO2, with humidified air. Cytosine arabinoside was added at 3 days in vitro (DIV) to produce a final concentration of 2 µM, and maintained for 2 days to halt non-neuronal cell division. The culture medium was changed after 5 DIV. Experiments were performed on cultures at 6-7 DIV.
All procedures involving experimental animals complied with regulations for the care and use of laboratory animals provided by the Animal Ethics Committee of Chonnam National University.
After washing with MEM (with Earle's salts) and adding 1 µM MK-801, the cultures were exposed to fluoxetine and various drugs for 24 hours in a serum-free culture medium. Each row of the 24-well plates contained four wells that received the same treatment. The four wells in the first row were treated with a sham wash, and 4 to 8 µl of the drug was added to the wells in the second to sixth rows, along with the culture media.
To evaluate cell survival, an MTT assay was performed according to a modification of the original procedure. Briefly, the tetrazolium salt MTT [3-(4,5-demethyl-2-thiazolyl)-2, 5-diphenyl-2H tetrazolium bromide; Sigma-Aldrich, St. Louis, MO, United States] was added to the cultures at a final concentration of 5 mg/mL. After incubation at 37℃ for 2 hours, the reaction was stopped by adding a lysis buffer containing 20% SDS and 50% N,N-dimethyl formamide, pH 4.7. Absorbance at 570 nm was monitored using a microplate reader (Molecular Devices, San Jose, CA, USA) after overnight incubation at 37℃.
For morphological evaluation of nuclei, SYTOX Green (Invitrogen, Carlsbad, CA, USA) staining was used. After fixing cells with 4% paraformaldehyde for 40 minutes at room temperature, the cells were permeabilized with 0.5% triton X-100 for 10 minutes, stained with 1 µM SYTOX Green for 15 minutes, and mounted with a SlowFade Antifade kit (Molecular Probes, Eugene, OR, USA). Changes in nuclear shapes and patterns were observed, and photographs were taken under a fluorescent microscope (Ex/Em=504/523 nm).
To examine DNA cleavage, cells were cultured in a 6-well plate and exposed to fluoxetine for various durations of times. The drug-treated cells were washed and lysed in a lysis buffer (0.5% Triton X-100, 5 mM Tris, 20 mM EDTA) overnight at 55℃. The lysates were extracted with chloroform, and the DNA in the aqueous layer was precipitated with ethanol following the addition of sodium acetate. The DNA was then collected by centrifugation, dried, and dissolved in TE buffer. The absorbance at 260 nm was measured in order to standardize the DNA concentration of all samples. Four micrograms of DNA was electrophoresed on a 1.5% agarose gel.
Intracellular ROS were respectively examined using 2, 7-dichlorofluorescin diacetate (DCF; Invitrogen, Carlsbad, CA, USA). Cell were treated with 10 µM DCF for 30 minutes, then treated with fluoxetine alone or in combination with bathocuproine disulfonic acid and deferoxamine. After treatment, ROS generation was monitored using a fluorescent multiwell plate reader (Fluoreskan Ascent FL, Labsystem, Vantaa, Finland) with excitation at 485 nm and emission at 530 nm. Each fluorescence value was obtained by subtracting the mean background value of the sham-treated control cultures. In some cases, the cultures were viewed using fluorescence microscopy and photographed.
Cells suspended in MEM were treated with 20 µM Phen Green FL, a fluorescent copper indicator, for 15 minutes. After dye loading, cells were washed three times with PBS. After treatment with 20 µM fluoxetine alone, or in combination with bathocuproine disulfonic acid and deferoxamine, the fluorescence of Phen Green FL was measured at an excitation wavelength of 492 nm and an emission wavelength of 517 nm using a fluorescent microscope.
Media for cell cultures was purchased from Gibco BRL (Rockville, MD, USA). HEPES (acid), glucose, NaHCO3, NaCl, KCl, MgCl2, CaCl2, NaOH, phenol red, trypsin, cytosine arabinoside, epidermal growth factor, sucrose, ascorbate, cycloheximide, Ca-EDTA, Zn-EDTA, EGTA, deferoxamine, bathocuproine disulfonic acid, and trolox were purchased from Sigma-Aldrich. N-benzyloxycarbonyl-Val-Ala-Asp-fluoromethylketone (z-VAD-FMK) was purchased from Calbiochem Corporation (San Diego, CA, USA). Brain-derived neurotrophic factor (BDNF) was purchased from Upstate Biotechnology (Hampton, NH, USA), and MK-801 was purchased from RBI (Natick, MA, USA). Fetal bovine and horse sera were purchased from Hyclone (Logan, UT, USA) and inactivated for 30 minutes at 55℃ before use.
To determine the effect of fluoxetine on cortical neuronal cell survival, mouse cortical near-pure neuronal cell cultures at 6-7 DIV were treated with various concentrations of fluoxetine (1-30 µM) for 24 hours. Unexpectedly, the addition of fluoxetine (3-30 µM) to the media for 24 hours induced neuronal death in a concentration-dependent manner (Fig. 1A). Treatment with 3 µM fluoxetine induced minor neuronal death (% cell viability=86±0.9%; n=8). Neuronal death increased with treatment of 10 µM, 15 µM, and 20 µM fluoxetine (% cell viability=77±2.3%, 62±3.9%, 41±3.0%, respectively; n=8). Treatment with 25 µM and 30 µM fluoxetine induced severe neuronal death (% cell viability 27±2.9% and 21±1.8%, respectively; n=8).
To examine whether apoptosis is involved in fluoxetineinduced neuronal death, DNA cleavage was assayed by agarose gel electrophoresis. Fluoxetine induced the cleavage of DNA into oligonucleosome fragments, manifested as ‘DNA laddering’, a hallmark of apoptosis. DNA laddering was clearly detected 24 hours after fluoxetine treatment (Fig. 1B). Furthermore, in the absence of fluoxetine, neurons exhibited normal cellular morphology, as visualized by the nucleic acid dye SYTOX Green, although treatment with fluoxetine induced nuclear chromatin condensation and nuclear fragmentation, other hallmarks of apoptosis (Fig. 1B).
The effects of some anti-apoptotic agents such as the protein synthesis inhibitor cycloheximide (CHX), BDNF, and the nonselective caspase inhibitor z-VAD-FMK on fluoxetine-induced neuronal death were examined. Neuronal viability (40±2.3%; n=12) was significantly increased in the presence of CHX (63±2.1%; n=12), BDNF (68±4.3%; n=8), and z-VAD-FMK (78±4.1%; n=8) (p<0.05) (Fig. 1C). These results suggest that the apoptotic process is involved in fluoxetine-induced neuronal death.
To investigate whether ROS were generated in the process of fluoxetine-induced neuronal death, we examined the effects of the antioxidants trolox (100 µM) and ascorbate (100 µM) on fluoxetine-induced neuronal death. As shown in Fig. 2A, trolox and ascorbate markedly restored neuronal survival from 43±1.8% (n=12) to 92±2.8% (n=8) and 80± 2.1% (n=8), respectively (p<0.01). Interestingly, treatment with all three divalent cation chelators, EGTA (69±3.9%; n=12), Zn-EDTA (74±1.6%; n=12), and Ca-EDTA (68±4.2%; n=12), markedly increased neuronal survival (p<0.01) (Fig. 2A). These findings suggest that extracellular Ca2+ and Zn2+ might not participate in fluoxetine-induced neuronal death. In light of this, candidate divalent ions are transition metals such as Fe2+ and Cu2+ because of their abundance in serum. First, we examined whether these transition metals contributed to ROS generation after exposure to fluoxetine. Exposure of cultures to fluoxetine for 8 hours markedly increased cellular DCF fluorescence, an indicator for superoxide, compared with sham-washed controls. Treatment with either trolox or bathocuproine disulfonic acid (a cell membrane impermeable copper chelator), markedly reduced DCF fluorescence, although treatment with deferoxamine (100 µM), an iron chelator, failed to reduce the fluorescence (Fig. 2B). This data demonstrates that ROS generation by fluoxetine is associated with extracellular copper ions. To determine the relationship between ROS generation and neuronal cell death by fluoxetine, we examined the effect of bathocuproine disulfonic acid and deferoxamine on fluoxetine-induced neuronal death, as shown in Fig. 2C. Treatment with bathocuproine disulfonic acid (300 µM, 1 mM) markedly increased neuronal survival, although deferoxamine (100 µM) did not affect neuronal survival.
We also examined changes in intracellular copper ion concentration using Phen Green FL, a cell permeable copper-selective fluorescent dye. When this dye binds with copper ions, its fluorescence intensity decreases (quenches).25 As shown in Fig. 3A, fluorescence was almost abolished by treatment with 10 µM CuCl2 for 1 hour, although it was not affected by treatment with 10 µM FeCl2. On the other hand, treatment with 20 µM fluoxetine significantly reduced fluorescence, and this was reversed by treatment with bathocuproine disulfonic acid, although not by treatment with deferexamine (Fig. 3). These results suggest that an influx of copper ions mediates fluoxetine-induced neuronal death.
In the present study, fluoxetine induced neuronal death in near-pure neuronal cultures in a concentration-dependent manner (Fig. 1A), and exposure of the cultures to 3 µM fluoxetine for 24 hours induced significant neuronal cell death. Since the therapeutic plasma concentration of fluoxetine showing clinical effects is reported to be around 1 µM,26 the neurotoxic concentration of fluoxetine in our culture system was comparable.
Some apoptotic features such as DNA laddering, chromatin condensation, and nuclear fragmentation were observed in fluoxetine-treated cultures (Fig. 1B). In addition to these features, all anti-apoptotic agents used in the present study, such as cycloheximide (a protein synthesis inhibitor), BDNF, and z-VAD-fmk (a pan-caspase inhibitor) significantly inhibited fluoxetine-induced death (Fig. 1C). This data demonstrates that fluoxetine induces neuronal apoptosis in cortical neuronal cultures, and this result is in agreement with the reports by Post et al.22 and Levkovitz et al.23
On the other hand, the prototypical antioxidants, trolox (a vitamin E analog) and ascorbate, almost restored neuronal cell viability (Fig. 2A). Interestingly, treatment with all three divalent cation chelators, EGTA, Zn-EDTA, and Ca-EDTA, markedly increased neuronal survival. As Zn-EDTA and Ca-EDTA chelate all divalent cations except Zn2+ and Ca2+, respectively, we can exclude extracellular Ca2+ and Zn2+ as mediators of fluoxetine-induced ROS generation and neuronal death. ROS generation after exposure to fluoxetine, measured by cellular DCF fluorescence, was significantly reduced by treatment with either trolox or bathocuproine disulfonic acid27 (a cell membrane impermeable copper chelator), although treatment with deferoxamine (an iron chelator) failed to reduce ROS generation (Fig. 2B). Furthermore, treatment with bathocuproine disulfonic acid markedly increased neuronal survival, although deferoxamine did not affect neuronal survival (Fig. 2C). This data demonstrates that ROS generation and neuronal cell death induced by fluoxetine are associated with extracellular copper ions.
We examined the changes in intracellular copper ion concentration using Phen Green FL, a cell permeable copper-selective fluorescent dye. When this dye binds with copper ions, its fluorescence intensity decreases (quenches).25 Fluoxetine induced fluorescence quenching was reversed by treatment with bathocuproine disulfonic acid and not by treatment with deferoxamine (Fig. 3). These results suggest that the influx of copper ions mediates fluoxetine-induced ROS generation and neuronal death. We could not find reports of any studies examining the relationship between fluoxetine and copper ions. Interestingly, Alsop and Wood28 reported that the combination of copper ions and fluoxetine showed the most toxic effect on the survival of zebra fish larva, amongst a variety of metal and pharmaceutical environmental contaminants.
Castro et al.29 reported that neocuprine, a well-known copper ion chelator, moved the copper ions from extracellular space to the cytoplasm. Based on this report, we propose that fluoxetine makes a complex with copper ions, and the complex gets across the plasma membrane and then copper ions are released in cytoplasm.
In conclusion, although the mechanisms regarding how fluoxetine introduces copper ions into neuronal cells must be delineated, we report for the first time that fluoxetine selectively moves copper ions into the cell, generates ROS, and ultimately induces neuronal cell death.
Figures and Tables
 | FIG. 1Fluoxetine-induced apoptotic neuronal death in mouse cortical cultures. (A) Concentration-dependent neurotoxic effects of fluoxetine measured at the end of a 24-hour exposure in neuronal cultures. Cell viability was measured by MTT assay. Each point and vertical bar represents the mean±SEM from 8-24 wells. (B-left) Electrophoretic laddering of neuronal cell DNA induced by 20 µM fluoxetine. DNA was isolated at 6 and 24 hours after exposure to 20 µM fluoxetine, and 4 µg was separated on an 1.5% agarose gel stained with ethidium bromide. (B-right) Fluorescent photomicrographs from typical representative fields (200×field) of cells taken after a 12-hour exposure to sham wash (Sham) or 20 µM fluoxetine (FLX). Arrows indicate the fragmented and condensed chromatin stained with SYTOX green. (C) Effects of treatment with 0.1 µg/mL cycloheximide (CHX), 100 nM BDNF, and 100 µM z-VAD-FMK (ZVAD) on the 20 µM fluoxetine-induced neuronal death at the end of a 24-hour exposure. The mean±SEM from 8-24 wells is shown. *Significantly different from the corresponding fluoxetine-treated control group (p<0.01). |
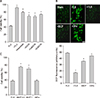 | FIG. 2Fluoxetine-generated ROS and -induced oxidative neuronal death associated with extracellular copper ions. (A) Effects of treatment with 100 µM trolox (TLX), 100 µM ascorbate, 1 mM EGTA, 1 mM Ca-EDTA, and 2 mM Zn-EDTA on the 20 µM fluoxetine-induced neuronal death at the end of a 24-hour exposure. The mean±SEM from 8-24 wells is shown. *Significantly different from the corresponding fluoxetine-treated control group (p<0.01). (B-top) Intracellular reactive oxygen species (ROS) generation by 20 µM fluoxetine and the effects of treatment with trolox (100 µM), bathocuproine disulfonic acid (1 mM, BCP), and deferoxamine (100 µM, DFe) on fluoxetine-induced ROS generation in mouse cortical cultures. Intracellular ROS were examined using 2,7-dichlorofluorescin diacetate (DCF). Cells were treated with 10 µM DCF for 30 minutes and then treated with fluoxetine alone or in combination with trolox, BCP, and DFe. After treatment, ROS generation was measured by a spectrophotometer with excitation at 485 nm and emission at 530 nm. (B-bottom) Each fluorescence value was obtained by subtracting the mean background value of sham-treated control cultures. The mean±SEM from 8-12 wells is shown. *Significantly different from the corresponding fluoxetine-treated control group (p<0.01). (C) Effects of bathocuproine disulfonic acid (BCP; 0.3 and 1 mM), deferoxamine (DFe; 100 µM), on the 20 µM fluoxetine-induced neuronal death at the end of a 24-hour exposure. The mean±SEM from 8-24 wells is shown. *Significantly different from the corresponding fluoxetine-treated control group (p<0.01). |
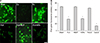 | FIG. 3Fluoxetine-induced copper ion influx into neurons. (A) Representative fluorescence photography showing fluorescence intensity changes by 20 µM fluoxetine (FLX), 10 µM CuCl2 (Cu), 10 µM FeCl2 (Fe), and the effect of 1 mM bathocuproine disulfonic acid (FLX+BCP) or 100 µM deferoxamine (FLX+DFe) on fluoxetine-induced quenching in cultured cortical cells treated with Phen Green FL dye. Phen Green FL fluorescence in cortical cultures with sham wash (Sham) or exposure to 20 µM fluoxetine without (FLX) or with the addition of 1 mM of bathocuproine disulfonic acid (BCP) or 100 µM deferoxamine (DFe) is apparent. Cells suspended in MEM were treated with 20 µM Phen Green FL for 15 minutes. After treatment, fluorescence of Phen Green FL was measured at an excitation wavelength of 492 nm and an emission wavelength of 517 nm using a fluorescent microscope. (B) Each fluorescence value was monitored in a fluorescent multiwell plate reader at an excitation wavelength of 492 nm and an emission wavelength of 517 nm. The mean±SEM from 8-12 wells is shown. *Significantly different from the corresponding sham wash-treated control group (p<0.01). |
References
1. Wong DT, Bymaster FP, Engleman EA. Prozac (fluoxetine, Lilly 110140), the first selective serotonin uptake inhibitor and an antidepressant drug: twenty years since its first publication. Life Sci. 1995; 57:411–441.

2. Pancrazio JJ, Kamatchi GL, Roscoe AK, Lynch C 3rd. Inhibition of neuronal Na+ channels by antidepressant drugs. J Pharmacol Exp Ther. 1998; 284:208–214.

3. Deák F, Lasztóczi B, Pacher P, Petheö GL, Kecskeméti V, Spät A. Inhibition of voltage-gated calcium channels by fluoxetine in rat hippocampal pyramidal cells. Neuropharmacology. 2000; 39:1029–1036.


4. Ohno Y, Hibino H, Lossin C, Inanobe A, Kurachi Y. Inhibition of astroglial Kir4.1 channels by selective serotonin reuptake inhibitors. Brain Res. 2007; 1178:44–51.


5. Nahon E, Israelson A, Abu-Hamad S, Varda SB. Fluoxetine (Prozac) interaction with the mitochondrial voltage-dependent anion channel and protection against apoptotic cell death. FEBS Lett. 2005; 579:5105–5110.


6. García-Colunga J, Awad JN, Miledi R. Blockage of muscle and neuronal nicotinic acetylcholine receptors by fluoxetine (Prozac). Proc Natl Acad Sci U S A. 1997; 94:2041–2044.



7. Peer D, Dekel Y, Melikhov D, Margalit R. Fluoxetine inhibits multidrug resistance extrusion pumps and enhances responses to chemotherapy in syngeneic and in human xenograft mouse tumor models. Cancer Res. 2004; 64:7562–7569.


8. Barygin OI, Nagaeva EI, Tikhonov DB, Belinskaya DA, Vanchakova NP, Shestakova NN. Inhibition of the NMDA and AMPA receptor channels by antidepressants and antipsychotics. Brain Res. 2017; 1660:58–66.


9. Brandes LJ, Arron RJ, Bogdanovic RP, Tong J, Zaborniak CL, Hogg GR, et al. Stimulation of malignant growth in rodents by antidepressant drugs at clinically relevant doses. Cancer Res. 1992; 52:3796–3800.

10. Spanová A, KovárXMLLink_XYZ H, Lisá V, Lukásová E, Rittich B. Estimation of apoptosis in C6 glioma cells treated with antidepressants. Physiol Res. 1997; 46:161–164.

11. Stopper H, Garcia SB, Waaga-Gasser AM, Kannen V. Antidepressant fluoxetine and its potential against colon tumors. World J Gastrointest Oncol. 2014; 6:11–21.



12. Serafeim A, Holder MJ, Grafton G, Chamba A, Drayson MT, Luong QT, et al. Selective serotonin reuptake inhibitors directly signal for apoptosis in biopsy-like Burkitt lymphoma cells. Blood. 2003; 101:3212–3219.


13. Liu KH, Yang ST, Lin YK, Lin JW, Lee YH, Wang JY, et al. Fluoxetine, an antidepressant, suppresses glioblastoma by evoking AMPAR-mediated calcium-dependent apoptosis. Oncotarget. 2015; 6:5088–5101.


14. Kamarudin MNA, Parhar I. Emerging therapeutic potential of anti-psychotic drugs in the management of human glioma: a comprehensive review. Oncotarget. 2019; 10:3952–3977.



15. Basso J, Miranda A, Sousa J, Pais A, Vitorino C. Repurposing drugs for glioblastoma: from bench to bedside. Cancer Lett. 2018; 428:173–183.

17. Lee HJ, Kim JW, Yim SV, Kim MJ, Kim SA, Kim YJ, et al. Fluoxetine enhances cell proliferation and prevents apoptosis in dentate gyrus of maternally separated rats. Mol Psychiatry. 2001; 6:610725–728.


18. Micheli L, Ceccarelli M, D'Andrea G, Tirone F. Depression and adult neurogenesis: positive effects of the antidepressant fluoxetine and of physical exercise. Brain Res Bull. 2018; 143:181–193.


19. Manev R, Uz T, Manev H. Fluoxetine increases the content of neurotrophic protein S100beta in the rat hippocampus. Eur J Pharmacol. 2001; 420:R1–R2.
20. Zarei G, Reisi P, Alaei H, Javanmard SH. Effects of amitriptyline and fluoxetine on synaptic plasticity in the dentate gyrus of hippocampal formation in rats. Adv Biomed Res. 2014; 3:199.



21. Chiou SH, Chen SJ, Peng CH, Chang YL, Ku HH, Hsu WM, et al. Fluoxetine up-regulates expression of cellular FLICE-inhibitory protein and inhibits LPS-induced apoptosis in hippocampus-derived neural stem cell. Biochem Biophys Res Commun. 2006; 343:391–400.


22. Post A, Crochemore C, Uhr M, Holsboer F, Behl C. Differential induction of NF-kappaB activity and neural cell death by antidepressants in vitro. Eur J Neurosci. 2000; 12:4331–4337.


23. Levkovitz Y, Gil-Ad I, Zeldich E, Dayag M, Weizman A. Differential induction of apoptosis by antidepressants in glioma and neuroblastoma cell lines: evidence for p-c-Jun, cytochrome c, and caspase-3 involvement. J Mol Neurosci. 2005; 27:29–42.


24. Hewett SJ, Csernansky CA, Choi DW. Selective potentiation of NMDA-induced neuronal injury following induction of astrocytic iNOS. Neuron. 1994; 13:487–494.


25. Tõugu V, Karafin A, Palumaa P. Binding of zinc(II) and copper(II) to the full-length Alzheimer's amyloid-beta peptide. J Neurochem. 2008; 104:1249–1259.


26. Amsterdam JD, Fawcett J, Quitkin FM, Reimherr FW, Rosenbaum JF, Michelson D, et al. Fluoxetine and norfluoxetine plasma concentrations in major depression: a multicenter study. Am J Psychiatry. 1997; 154:963–969.


27. Mohindru A, Fisher JM, Rabinovitz M. Bathocuproine sulphonate: a tissue culture-compatible indicator of copper-mediated toxicity. Nature. 1983; 303:64–65.






 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download