Abstract
The macrophage displays functional and phenotypic diversity, which appears, in no small part, to stem from the ability of macrophages to adapt functionally to changes in their tissue microenvironment. Here, we describe the differential activity of peritoneal macrophages with or without the presence of thioglycollate (TG), an inflammatory drug that encouraged the recruitment of macrophages, during aging. The peritoneal-resident macrophages dramatically reduced in phagocytosis and pro-inflammatory cytokines secretion with aging, whereas the functions of macrophages recruited by TG were not significantly changed with aging. These results suggest that macrophages may be changed by their environment in advanced age, and could provide possible explanations for the controversial results regarding differential changes in macrophages in other papers.
Aged individuals can have a significantly higher basal inflammatory state, where circulating levels of pro-inflammatory cytokines lead to chronic tissue inflammation and age-associated diseases.12 Chronic inflammation affects tissue injury and attempts at repair can compound the problem, leading to tissue remodeling and dysfunction.3 Macrophage functions have been implied in chronic inflammatory processes as part of the pathological connection between chronic inflammation and age-associated diseases.45
Macrophages in the immune system act as sensors for pathogens, indicated as “dangerous”, by detecting pathogen-associated molecular patterns (PAMPs) and endogenous signals released from damaged tissue through their pathogen recognition receptors (PRRs).67 Toll-like receptors (TLRs) are a subset of these PRRs that respond to PAMPs and certain endogenous proteins associated with “danger” and stress. They can specifically recognize PAMPs, microbes, microbial products, and pharmaceutical compounds. Stimulation of TLRs by their specific ligands results in the secretion of pro-inflammatory cytokines such as interleukin (IL)-6, which initiate an inflammatory response to clear the invading organism. Macrophages phagocytose the invading organisms, initiate an inflammatory response that recruits neutrophils and natural killer cells, and facilitate the maturation, differentiation, and migration of dendritic cells.89
Macrophages are divided into pro-inflammatory M1 macrophages or anti-inflammatory M2 macrophages.10 Macrophages are differentiated to M1 macrophages exposed by specific Th1 cytokines of TLR ligand. M1 macrophages secrete pro-inflammatory cytokines lead to inflammatory responses and produce cellular reactive oxygen species (ROS). By contrast, M2 macrophages are differentiated by Th2 cytokines, including IL-4.11 It is well known that M2 macrophages promote the resolution of inflammation and fibrosis through the secretion of anti-inflammatory cytokines.12 The different functions of M1 and M2 macrophages have been demonstrated in various tissues relevant to aging-related diseases.
Advanced age affects a number of macrophage functions, including anti-bacterial defense by phagocytosis, inflammatory responses through secretion of cytokines, immune cells infiltration, and wound healing.13 While levels of pro-inflammatory cytokines, specially IL-6, are elevated in serum isolated from aged animals and humans,1415 the production of various pro-inflammatory cytokines by peritoneal macrophages in mice, and rats decrease with age. The secretion of IL-6, tumor necrosis factor (TNF)-α, IL-1β, and chemokines from aged rodent macrophages by the stimulation of lipopolysaccharide (LPS) is lower than those of young rodents. There remains some controversy for the decline of pro-inflammatory responses by LPS stimulation. Renshaw et al.16 reported that the expression of TLRs in macrophages declined with advancing age, lead to the decrease of cytokine production upon stimulation of LPS. In contrast, Bohehmer et al.17 reported that TLR expression was not affected by advanced age and that reduced cytokine production was due to impairment of LPS-induced phosphorylation of the mitogen-activated protein kinases (MAPKs).
Previously, we reported that flagellin-dependent TLR5 activation is preserved in aging macrophages, by stabilization with caveolin-1. Furthermore, we proposed that flagellin may be a promising immune activator for pneumococcal vaccine development for the elderly.18 For this study, we screened TLRs expression and activation using peritoneal-resident-macrophages without inflammatory stimulation. However, other studies have used peritoneal macrophages after inflammatory stimulation for recruiting large numbers of macrophages into the peritoneum. In this study, we hypothesized that the microenvironment resulting from inflammatory stimulation or aging might affect tissue macrophage functions. To elucidate this hypothesis, we used two types of mouse peritoneal macrophages, with or without thioglycollate (TG)-treatment, and compared their specific functions with advanced age.
Eight-week-old female C57BL/6J mice were purchased from The Jackson Laboratory (Bar Harbor, ME, USA), and 24-month-old female C57BL/6J mice were provided from the Korea Basic Science Institute (Daejeon, Korea). All mice were maintained in a specific pathogen-free animal facility at the Clinical Vaccine R&D Center of Chonnam National University and conducted in accordance with the guidelines of the Animal Care and Use Committee of Chonnam National University.
Salmonella protein (Sal-P) was extracted from bacteria using lysis buffer [50 mM Tris-HCl (pH 7.4), 1 mM ethylenediaminetetraacetic acid (EDTA), 1 mM phenylmethylsulfonyl fluoride (PMSF)]. LPS (Escherichia coli O127:B8) was purchased from Sigma-Aldrich (St. Louis, MO, USA). Vibrio vulnificus major flagellin (Vv-FlaB) recombinant proteins were obtained from Professor Joon Haeng Rhee, Chonnam National University. Enzyme-linked immunosorbent assay (ELISA) kits for IL-6 (#14-7061-68), IL-1β (#14-7012-67), IL-10 (#88-7105-88), and TNF-α (# 88-7324-86) were purchased from eBioscience (San Diego, CA, USA). Cell culture-associated reagents were purchased from Gibco-BRL (Grand Island, NY, USA). Other biochemical reagents were purchased from Sigma-Aldrich.
Tissue-resident and TG-elicited macrophages were isolated from the peritoneum in young and old mice. To harvest peritoneum-resident macrophages, peritoneal lavage with 10 mL of phosphate-buffered saline (PBS) containing 10% fetal bovine serum (FBS) was performed. The isolated peritoneal cells were seeded in culture dishes for 12 h. After washing out, the adherent cells were cultured in RPMI-1640 medium supplemented with 10% FBS and 1% penicillin/streptomycin. To harvest TG-elicited macrophages, each mouse was intraperitoneally injected with 2.5 mL of 3% TG. Three days later, peritoneal exudates were collected and seeded in culture dishes for 12 h. Cell culture procedures were the same as described above.
To analyze the phagocytic abilities of each type of macrophage, we used Salmonella typhimurium (S. typhimurium) (14028S). S. typhimurium was cultured in Luria-Bertani (LB) medium at 37℃ for overnight. Bacteria were next inoculated into fresh cultures and grown for 4 h at 37℃ with agitation at 200 rpm. Peritoneum-resident and TGelicited peritoneal macrophages were seeded (1×105 cells) in 96-well culture plates and grown in RPMI-1640 medium supplemented with 10% FBS at 37℃ in a 5% CO2 incubator, and infected with S. typhimurium (multiplicity of infection 1:10) for 30 min. After 30 min, infected cells were washed with PBS three times and then afresh medium containing gentamycin (10 µg/mL) was added and left for 30 min to kill residual extracellular bacteria. Thereafter, the infected cells were lysed with PBS contained with 0.05% Triton X-100. Cell lysate were harvested and then plated on LB agar plates. Colonies were allowed to develop for 16 h before counting. Assays were conducted in triplicate.
Peritoneum macrophages were stimulated by LPS (5 µg/mL), Sal-P (10 µg/mL), and Vv-FlaB (100 ng/mL) in serum-free medium. After stimulation for 12 h, cell culture supernatants were collected and immediately assayed for IL-6, IL-1β, IL-10, and TNF-α levels by ELISA. ELISA was performed in accordance with the manufacturer's protocols (ebioscience).
Peritoneum macrophages were stimulated with serumfree medium supplemented with LPS (5 µg/mL). After stimulation for 12 h, cells were suspended in staining buffer (eBioscience, # 00-4222) and stained with F4/80-FITC (#11-4801) (ebioscience), CD11b-PE (#12-0112-82) (eBioscience), and CD206-Alexa Fluor 647 (#141712) (BioLegend, San Diego, CA, USA) for 30 min in darkness at 4℃. Stained cells were washed twice in staining buffer and finally suspended in 1% paraformaldehyde in PBS. Flow cytometry data were acquired and analyzed using a FACS Calibur (BD Biosciences, San Diego, CA, USA).
Statistical analysis was conducted using GraphPad Prism 5 software (GraphPad, Inc., San Diego, CA). Differences between experimental groups were analyzed by Mann-Whitney U-test and were considered significant for p values<0.05. Data is shown to be independent in experiments at least three times and presented as the mean ± SEM.
Since peritoneal macrophages are generally isolated by collecting cells either with or without injecting intraperitoneally, any controversy regarding age-related data from macrophages may stem from the different macrophage conditions. To establish differences between two peritoneal macrophage models, we isolated peritoneumresident and TG-elicited macrophages from young mice and compared cell numbers and phagocytosis. As expected, the total number of macrophages after TG treatment was higher than for peritoneum-resident macrophages (Fig. 1A). Next, we examined macrophage subpopulations from peritoneum with or without TG treatment. Since expression of murine F4/80 is an insufficient marker to discriminate between monocyte-derived macrophages and tissue-resident macrophages, the F4/80+ macrophages were gated and we analyzed subpopulations of M1 or M2 macrophages with specific markers. The macrophages were broadly categorized into two main subtypes, CD206− pro-inflammatory M1 and CD206+ anti-inflammatory M2.19 Most of the peritoneum-resident macrophages were M1-like macrophages (CD 11b+/CD206−), but TG treatment differentiated to M2-like macrophages (CD 11b+/CD206+) (Fig. 1B). There were no remarkable changes of cell population in LPS treatment.
Since M2 macrophages are known to be essential for parasite clearance and to promote resolution of inflammation,1220 we tested phagocytic functions by counting intracellular Salmonella bacteria after infection. Phagocytic activity was also increased in TG-elicited macrophages (Fig. 1C). LPS-dependent pro-inflammatory cytokine secretions were markedly higher in peritoneum-resident macrophages than in TG-elicited macrophages (Fig. 1D). However, anti-inflammatory cytokine IL-10 was increased by TG treatment as well as LPS stimulation in TG-elicited macrophages. These results indicate that peritoneum-resident macrophages showed M1-like phenotypes, and that TG-dependent inflammatory stimuli might induce the polarization from M1- to M2-like macrophages in the peritoneum.
Since macrophages are infiltrated into aged tissues and lead to age-associated chronic inflammation21 and many papers have discussed the controversy surrounding agedependent functional alterations in macrophages, we compared the functions of peritoneum-resident and TG-elicited macrophages isolated from old mice (24 months or older). The total number of aged macrophages was increased by TG-treatment to similar levels as in young macrophages (Fig. 2A). In aged peritoneum, M2-like macrophage populations (CD 11b+/CD206+) were also increased by TG treatment and slightly increased by LPS stimulation (Fig. 2B). While M2-like macrophages showed higher activity levels, including parasite clearance and anti-inflammatory functions, secreting cytokine like young peritoneum macrophages, we found no difference in LPS-dependent IL-10 secretion (Fig. 2C, D). Peritoneum-resident aged macrophages showed similar phenotypes to young macrophages, including M1-like macrophages (CD11b+/CD206−) and pro-inflammatory responses by LPS stimulation (Fig. 2B, D). As shown in Fig. 1, 2, the population of M2 macrophages in peritoneum increased with age (from 6.8% to 21.02%) and TG-treatment also induced M2 macrophages (from 21.02% to 53.72%) in aged peritoneum, suggesting that inflammatory drugs or age-dependent chronic inflammation might induce M2 macrophages.
To elucidate the age-dependent functional differences between M1 and M2 macrophages in the peritoneum, we compared total cell numbers and phagocytotic functions. Other studies have reported that the numbers of innate immune cells, including macrophages and neutrophils, are maintained during the aging process.79 Here we also obtained greater numbers both of M1- or M2-like macrophages in peritoneum from old mice than from young mice (Fig. 3A). However, phagocytotic activity was significantly decreased in peritoneum-resident M1-like macrophages from old mice (Fig. 3B). Unlike peritoneum-resident macrophages, phagocytotic function was well maintained in TG-elicited M2-like macrophages from old mice (Fig. 3B). These results provide support for the idea that macrophage phenotype-dependent functions differ with age.
Next, since it is well known that M1 macrophages strongly recognize PAMPs by TLRs, leading to pro-inflammatory cytokine secretion,2223 we assessed pro-inflammatory cytokine secretion after stimulation with Salmonella extracts, which activate various TLRs, LPS for TLR4, or flagellin for TLR5 activation in macrophages from young and old mice. Although the total number of M1-like peritoneumresident macrophages was greater in old mice, their ability to recognize PAMPs decreased (Fig. 4). Salmonella extracts and LPS-dependent secretion of IL-1β, IL-6, and TNF-α in peritoneum M1-like macrophages from old mice were lower compared to cells from young mice. M1-like macrophages from old mice also responded to stimulation, but cytokine secretion was lower compared to cells from young mice. Although flagellin-dependent cytokine secretion was lower than for cells exposed to Salmonella extracts or LPS, the responses were well maintained in M1-like macrophages from young or old mice. This indicates that peritoneum-resident M1-like macrophages in old mice may have reduced recognition of PAMPs by TLRs, leading to reduced cytokine secretion. However, it is known that flagellin-dependent TLR5 activity is maintained in peritoneum-resident macrophages in old mice.18 As expected, both young and old TG-elicited M2-like macrophages showed low levels of proinflammatory cytokine secretion compared to peritoneumresident macrophages (Fig. 4). These results suggest that peritoneum-resident M1-like macrophages showed decreased ability in pathogen recognition activity with age. Furthermore, peritoneal macrophages were differentiated from M1- to M2-like macrophages by chronic or drug-dependent inflammation and their phagocytotic activity was preserved with age.
The macrophage phenotypes display functional plasticity, which stem from the ability of macrophages to adapt functionally to changes in their tissue environment.2425 This functional plasticity plays a critical role in the ability of macrophages to respond to infection or tissue abnormalities and to contribute to the clearance of damaged tissue and invading microorganisms, as well as to further recruitment of the adaptive immune system.89
To determine whether their environments differentially activate macrophages, we compared two types of peritoneal macrophages isolated from young mice. Injection of TG into the peritoneum may induce inflammatory responses, leading to the recruitment of macrophages to this region. Seeing as TG treatment can yield large numbers of macrophages, TG-elicited macrophages have been used as a representative model of macrophage-related research. However, since inflammatory responses can affect macrophage activation, we should consider their biological status and activity. Our study found differences in activity between peritoneum-resident and TG-elicited peritoneal macrophages. While peritoneum-resident macrophages showed M1-like phenotypes with specific markers and were sensitive to stimulation with PAMPs (Salmonella extracts, LPS, and flagellin), TG-elicited peritoneal macrophages showed M2-like phenotypes with strong phagocytotic function following Salmonella infection. These results suggest that peritoneum-resident M1-like macrophages may have welldeveloped PRRs, including TLRs, resulting in rapid recognition of infection by pathogens and the warning of other immune systems. Thus M2-like macrophages recruited to the peritoneum from other regions by inflammatory stimulation, including TG treatment, may acquire strong phagocytic activity, leading to rapid removal of pathogens or dead cells for wound healing in the peritoneum.
Many biological properties of macrophages are affected by advanced age, but the impact of aging on the immune system, including macrophages, remains unclear. While some reports have shown decreased phagocytotic functions of macrophages in aged humans and mice,26 other studies using aged rats have found the opposite27 or no age-related effects.28 Thus, there remains controversy regarding TLR-dependent cytokine production in advanced aging.1617 These differences may reflect differences in the activation state and source of macrophages, or particular experimental conditions.29 To understand the differences using macrophage models of aging, we compared specific macrophage functions in young and old mice. Peritoneum-resident M1-like macrophages from old mice showed decreased TLR-induced cytokine production following treatment with Salmonella extracts and LPS, results similar to previously reported data.30 Furthermore, flagellin-dependent TLR5 activity was preserved in old peritoneum-resident M1-like macrophages, suggesting that the aging process differentially affects PPRs expressed on the surface of innate immune cells. Unlike peritoneum-resident macrophages, TG-elicited M2-like macrophages from old mice showed similar phagocytic activity after Salmonella infection and low levels of cytokine production following stimulation with Salmonella extracts, LPS, and flagellin. This indicates that drug- or age-dependent inflammation might induce differentiation from M1- to M2-like macrophages in the peritoneum, and that aged M2-like macrophages preserve phagocytic activity for the removal of pathogens or dead cells for tissue remodeling.
The significance of our results is emphasized by the finding that activity differs between tissue-resident and TG-elicited macrophages in the peritoneum between young and old mice. Given the above discussion on the functional adaptability of macrophages to environmental changes, and evidence that age-related changes in macrophage function may be reversible rather than intrinsic, we suggest that targeting regulatory factors might, at least transiently, restore the inflammatory and pro-immunogenic functions of macrophages in the elderly.
Figures and Tables
 | FIG. 1Differential activation between tissue-resident and TG-elicited peritoneal macrophages from young mice. Peritoneum-resident (Mϕ)- and TG-elicited peritoneum macrophages (TG-Mϕ) were isolated from young mice (Y, 8 weeks old). Total cell numbers (n=5/each group) were counted (A), and phagocytotic activity (n=5/each group) was quantified (B). The ratio for Mϕ was arbitrarily defined as 100%, and the ratios for TG-Mϕ were expressed as relative values. (C) The cell surface expression of CD11b (M1 polarization marker) and CD206 (M2 polarization marker) in a F4/80-gated population of peritoneum-resident and TG-elicited peritoneum macrophages (n=5/each group) was analyzed by flow cytometry. (D) Cells (n=5/each group) were stimulated with LPS (5 mg/mL). IL-6, TNF-α, and IL-1β, and IL-10 levels were analyzed by ELISA. *p<0.05; **p<0.01. #p<0.05; ##p<0.01 compared with non-treated and LPS-treated macrophages. LPS: lipopolysaccharide, TG: thioglycollate. |
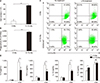 | FIG. 2Differential activation between tissue-resident and TG-elicited peritoneal macrophages from old mice. Mϕ and TG-Mϕ were isolated from old mice (O, 24 months). Total cell numbers (n=5/each group) were counted (A), and phagocytotic activity (n=5/each group) was quantified (B). (C) The cell surface expression of CD11b and CD206 in a F4/80-gated population of Mϕ and TG-Mϕ (n=5/each group) was analyzed by flow cytometry. (D) Cells (n=5/each group) were stimulated with LPS (5 µg/mL). IL-6, TNF-α, IL-1β, IL-10 levels were analyzed by ELISA. *p<0.05; **p<0.01; ***p<0.001. #p<0.05 compared with non-treated and LPS-treated macrophages. NS indicates not significant. |
 | FIG. 3Comparison of functional alterations in peritoneum-resident macrophages from young and old mice. After isolation of Mϕ and TG-Mϕ from Y- and O-mice, total cell numbers (n=5/each group) were counted (A), and phagocytotic activity (n=5/each group) was quantified (B). **p<0.01; ***p<0.001. NS indicates not significant. |
 | FIG. 4Age-associated functional alterations of peritoneum-resident macrophages. After isolation of Mϕ and TG-Mϕ from Y- and O-mice, cells (n=5/each group) were stimulated with Salmonella extract proteins (Sal-P) (10 µg/mL), LPS (5 µg/mL), and Vv-FlaB (100 ng/mL). IL-6 (A), TNF-α (B), and IL-1β (C) levels were analyzed by ELISA. *p<0.05; NS indicates not significant. Vv-FlaB: Vibrio vulnificus major flagellin recombinant proteins. |
ACKNOWLEDGEMENTS
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (No. NRF-2017R1E1A2A02081815 and NRF-2017R1E1A1A01074674), and this research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (grant number NRF-2018R1D1A1B051207).
References
1. Rea IM, Gibson DS, McGilligan V, McNerlan SE, Alexander HD, Ross OA. Age and age-related diseases: role of inflammation triggers and cytokines. Front Immunol. 2018; 9:586.

2. Michaud M, Balardy L, Moulis G, Gaudin C, Peyrot C, Vellas B, et al. Proinflammatory cytokines, aging, and age-related diseases. J Am Med Dir Assoc. 2013; 14:877–882.

3. Karin M, Clevers H. Reparative inflammation takes charge of tissue regeneration. Nature. 2016; 529:307–315.



4. Oishi Y, Manabe I. Macrophages in age-related chronic inflammatory diseases. NPJ Aging Mech Dis. 2016; 2:16018.

5. Licastro F, Candore G, Lio D, Porcellini E, Colonna-Romano G, Franceschi C, Caruso C. Innate immunity and inflammation in ageing: a key for understanding age-related diseases. Immun Ageing. 2005; 2:8.

6. O'Neill L. Toll-like receptors and the danger hypothesis. Trends Immunol. 2001; 22:421.
8. Geissmann F, Manz MG, Jung S, Sieweke MH, Merad M, Ley K. Development of monocytes, macrophages, and dendritic cells. Science. 2010; 327:656–661.



9. Murray PJ, Wynn TA. Protective and pathogenic functions of macrophage subsets. Nat Rev Immunol. 2011; 11:723–737.



10. Italiani P, Boraschi D. From monocytes to M1/M2 macrophages: phenotypical vs. functional differentiation. Front Immunol. 2014; 5:514.

11. Chawla A, Nguyen KD, Goh YP. Macrophage-mediated inflammation in metabolic disease. Nat Rev Immunol. 2011; 11:738–749.



12. Martinez FO, Helming L, Gordon S. Alternative activation of macrophages: an immunologic functional perspective. Annu Rev Immunol. 2009; 27:451–483.


13. Stout RD, Suttles J. Immunosenescence and macrophage functional plasticity: dysregulation of macrophage function by age-associated microenvironmental changes. Immunol Rev. 2005; 205:60–71.



14. Franceschi C, Bonafè M, Valensin S, Olivieri F, De Luca M, Ottaviani E, et al. Inflamm-aging. An evolutionary perspective on immunosenescence. Ann N Y Acad Sci. 2000; 908:244–254.


15. Saurwein-Teissl M, Blasko I, Zisterer K, Neuman B, Lang B, Grubeck-Loebenstein B. An imbalance between pro- and anti-inflammatory cytokines, a characteristic feature of old age. Cytokine. 2000; 12:1160–1161.


16. Renshaw M, Rockwell J, Engleman C, Gewirtz A, Katz J, Sambhara S. Cutting edge: impaired Toll-like receptor expression and function in aging. J Immunol. 2002; 169:4697–4701.


17. Boehmer ED, Goral J, Faunce DE, Kovacs EJ. Age-dependent decrease in Toll-like receptor 4-mediated proinflammatory cytokine production and mitogen-activated protein kinase expression. J Leukoc Biol. 2004; 75:342–349.


18. Lim JS, Nguyen KC, Nguyen CT, Jang IS, Han JM, Fabian C, et al. Flagellin-dependent TLR5/caveolin-1 as a promising immune activator in immunosenescence. Aging Cell. 2015; 14:907–915.



19. Jeong JH, Kim K, Lim D, Kim KH, Kim HS, Lee S, et al. Microvasculature remodeling in the mouse lower gut during inflammaging. Sci Rep. 2017; 7:39848.

20. Tabas I. Macrophage death and defective inflammation resolution in atherosclerosis. Nat Rev Immunol. 2010; 10:36–46.


21. Sarkar D, Fisher PB. Molecular mechanisms of aging-associated inflammation. Cancer Lett. 2006; 236:13–23.

22. Jiménez-Dalmaroni MJ, Gerswhin ME, Adamopoulos IE. The critical role of toll-like receptors--from microbial recognition to autoimmunity: a comprehensive review. Autoimmun Rev. 2016; 15:1–8.


23. Wang N, Liang H, Zen K. Molecular mechanisms that influence the macrophage m1-m2 polarization balance. Front Immunol. 2014; 5:614.

24. Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008; 8:958–969.



25. Gordon S, Plüddemann A, Martinez Estrada F. Macrophage heterogeneity in tissues: phenotypic diversity and functions. Immunol Rev. 2014; 262:36–55.



26. Fietta A, Merlini C, De Bernardi PM, Gandola L, Piccioni PD, Grassi C. Non specific immunity in aged healthy subjects and in patients with chronic bronchitis. Aging (Milano). 1993; 5:357–361.


27. Hilmer SN, Cogger VC, Le Couteur DG. Basal activity of Kupffer cells increases with old age. J Gerontol A Biol Sci Med Sci. 2007; 62:973–978.

28. Miller AP, Xing D, Feng W, Fintel M, Chen YF, Oparil S. Aged rats lose vasoprotective and anti-inflammatory actions of estrogen in injured arteries. Menopause. 2007; 14:251–260.






 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download