Abstract
Scavenger receptors typically bind to multiple ligands on a cell surface, including endogenous and modified host-derived molecules and microbial pathogens. They promote the elimination of degraded or harmful substances such as non-self or altered-self targets through endocytosis, phagocytosis, and adhesion. Currently, scavenger receptors are subdivided into eight classes based on several variations in their sequences due to alternative splicing. Since recent studies indicate targeting scavenger receptors has been involved in cancer prognosis and carcinogenesis, we will focus on the current knowledge about the emerging role of scavenger receptor classes A to E in cancer progression.
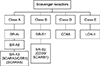
Scavenger receptors bind to numerous ligands on a cell surface, such as gram-positive/negative bacterial cell-wall components, microbial pathogens, and endogenous and modified host-derived molecules.1 They are considered surface receptors and not only remove toxic substances derived from outer cells and waste materials in various cells, but secrete various inflammatory cytokines, followed by accelerating host immune responses. In this article, we focus on the current knowledge about the emerging role of scavenger receptor classes A to E (Table 1; Fig. 1) in cancer prognosis and carcinogenesis.
The Sra gene located on chromosome 8p22 is closely associated with multiple tumor susceptibility phenotypes, and its genetic variations may be associated with enhanced susceptibility of prostate cancer.2 As the polymorphisms of various genes involved in the response of inflammatory regulation have been associated with higher cancer risk,3 a polymorphism of the Sra gene located in the 5′ untranslated region was also reported to increase lung cancer risk through down-regulated SRA expression,2 suggesting that the analysis of Sra polymorphisms may be a relevant scientific technique to evaluate the risk of cancer prior to disease onset of possible patients.
Scavenger receptor class A type I and type II (SR-AI and SR-AII) were found to be alternatively spliced products of a single gene in macrophage.456 The SR-A as a phagocytic pattern recognition receptor (PRR) is primarily expressed on tissue macrophages and dendritic cells (DCs).78 The class A scavenger receptors are involved in host biological functions such as host defense, the maintenance of tissue homeostasis by clearance of apoptotic cells, antigen presentation, and pathogenesis of neurodegenerative disorders.9 It has also been reported that the receptors could serve as PRRs for innate immunity based on their binding activities with broad ligands including a number of heat-shock proteins (HSPs) such as Hsp110, Grp94, and Grp170.101112
Scavenger receptor class A member 3 (SCARA3), which is also known as cellular stress response 1 (CSR1)/SR-A3, is associated with a high metastasis of prostate cancer. It was reported that the Scara3 gene was frequently downregulated and methylated in prostate cancer tissue samples.13 Since tumor growth and invasion was inhibited by the overexpression of SCARA3 in prostate cancer cells, SCARA3 may have a potent role in tumor suppressor in prostate cancer.1314 However, the overexpression of SCARA3 resulted in correlation with disease progression and recurrence in ovarian carcinoma and primary peritoneal carcinoma, 15 indicating that SCARA3-associated cancer therapeutic approaches may not yet be clear, and need more future studies in various cancer models.
The lower level of SCARA5 expression was reported in hepatocellular carcinoma (HCC) cells, and enhanced expression of SCARA5 resulted in suppression of its tumorigenesis and metastasis through the focal adhesion kinase (FAK)-Src-p130Cas signaling pathway.1617 Furthermore, upregulation of SCARA5 expression could be closely associated with STAT3 (signal transducer and activator of transcription 3) inactivation and low levels of STAT3-regulated genes, which is implicated in tumor progression and metastasis of breast cancer,18 indicating that SCARA5 may be a therapeutic agent for potential cancer control.
SR-B1, also known as SCARB1, modulates cholesterol metabolism in the liver, adrenal glands, and gonads through mediating the transportation of various cholesterols such as modified low-density lipoprotein (LDL), native high-density lipoprotein (HDL), and very low-density lipoprotein (VLDL).1920 Since there are several reports about low levels of HDL being a marker of cancer development and prognosis,2122 SCARB1 being a responsible receptor for internalization of HDL cholesteryl esters (HDL-CE) gets a lot of attention as a way to prevent cancer development. The upregulated expression of SR-B1 as a selective uptake of HDL-CE was detected in breast cancer, prostate cancer, ovarian cancer, and hepatoma cells.23242526
SR-B2 (CD36) is one of the best-known scavenger receptors, and was initially reported as a transmembrane glycoprotein that contains several post-translational modification sites. It plays a role as a binding receptor to diverse ligands, including apoptotic cells, thrombospondin (TSP).27 It also plays an important role in controlling tumor neovascularization through binding to TSP-1/2, the endogenous inhibitor of angiogenesis,28 and in contributing to phagocytosis during the resolution phase of ischemic stroke in mice.29 It was also reported that SR-B2, as a linking lipid to the NLRP3 inflammasome, has a biological function of regulating inflammatory processes in atherosclerosis and Alzheimer's disease.30
Furthermore, the induction of intracellular signaling cascades involving the mitogen-activated protein kinase (MAPK) p38 and c-Jun N-terminal kinase by CD36 binding to TSP-1/2 activates proapoptotic signals, including caspase 3 cleavage and induction of Fas/Fas-ligand.3132 Downregulated expression of CD36 activates metastasis of colon cancer, breast cancer, and ovarian cancer. It was reported that lower levels of CD36 expression were detected in highly aggressive breast tumor than its less aggressive cells.33 The increased metastatic potential may be induced by the low capacity of CD36 binding to collagen in the extracellular matrix (ECM), followed by reduced tumor cell adhesion to ECM.33 It was also reported that the downregulated expression of CD36 is closely associated with the pathologic changes in breast cancer and mammary gland hyperplasia, impaired adipocyte differentiation, and excessive ECM deposition.34 These studies suggest a therapeutic approach based on appropriate modulation of CD36 expression to prevent the aggressive progression of breast cancer.
CD68, as a glycosylated type I membrane protein, is predominantly expressed in macrophages, dendritic cells, and osteoclasts.3536 It was reported that CD68 is highly expressed in bone marrow-derived monocyte/macrophages, and closely associated with the entire stage of chronic liver injury.37 CD68 is also known to be a pan-macrophage marker for the tumor-associated macrophages (TAMs) detected in carcinogenesis.38 The CD68 (+) TAMs could be a unique marker for the prognosis of patients with oral squamous cell carcinoma (OSCC).38 Besides, it was reported that the high level of CD68 (+) TAMs was detected in worse breast cancers, and its lower levels were associated with improved metastasis-free survival rates in human breast cancer.39 Recent studies have indicated that a high level of CD68 (+) macrophages could also be a prognostic marker in nonmetastatic breast cancer.40 Increased levels of CD68 (+) TAMs were also reported to be accompanied by upregulated stromal and serum levels of VEGF by radiotherapy, which is related to an angiogenic tumor growth and metastasis in breast cancer.41 Although CD68 has been used as a prognostic pan-macrophage marker for carcinogenesis, there are still many inconsistent results through unknown mechanisms.
Lectin-like oxidized LDL receptor 1 (LOX-1) or oxidized low-density lipoprotein receptor 1 (OLR1) is known to bind to oxLDL, advanced glycation end products (AGEs), and apoptotic cells as a scavenger receptor class E, and primarily expressed in endothelial cells, cardiomyocytes, smooth muscle cells, B cells, macrophages, DCs, and vasculaturerich organs.4243 Upregulated LOX-1 expression was recently reported in a mechanistic connection between cellular transformation and atherosclerosis, and several types of cancers.42 The LOX-1 upregulation resulted in contributions to cellular transformation by stimulation of inflammatory cytokines such as IL-6, and IL-8, and hypoxia-regulated proteins such as VEGF and anhydrase 9 in a NF-κB dependent manner.44 It was also reported that LOX-1 could be an oncogene induced by NF-κB target genes responsible for proliferation, migration and inhibition of apoptosis in breast cancer.45 These studies indicate that the inhibition of LOX-1 expression could be a promising therapeutic approach in atherosclerosis and tumors. Recently, Kumari et al.46 developed novel LOX-1 inhibitors to block the interaction of LOX-1 with oxLDL to possibly prevent plaque formation in arteries and the initiation of atherosclerosis. It was also reported that LOX-1 is involved in host T cell-mediated immune responses, in which it could bind to PAMPs, followed by activation of dendritic cells (DCs) through collaborating with TLR2 and Hsp60/70.47 Furthermore, the treatment with anti-LOX-1 increased the secretion of MCP-1, MIP-1α, and IL-8, and the levels of HLLA-DR and CD86 in human dendritic cells. Interestingly, B cell activating factor (BAFF) and a proliferation inducing ligand (APRIL) in anti-LOX-1-activated DCs could promote humoral responses by inducing class switch and by promoting the generation of plasmablasts, indicating that LOX-1 expression in DCs could be a novel therapeutic target for increased cellular and humoral immunities as well as a key prognostic marker for promoted immune responses.48
Recently, there are not only numerous effective treatments for various cancers with scavenger receptor-targeted therapeutic agents, but the relative contribution of various scavenger receptors functionally activates anti-cancer immunity through the molecular basis of how ligand-specific interaction between scavenger receptors and its various coreceptors. However, further studies are required to elucidate more detailed influences associated with the outcomes of engaging scavenger receptors in various cancers as well as homeostatic and pathological states. A better understanding of the pathophysiological and immunological functions of scavenger receptors will facilitate the development of rational therapeutic approaches targeting of drug delivery and immune surveillance, which are expected to lead to improved outcomes in cancer treatment.
Figures and Tables
References
1. Matsumoto A, Naito M, Itakura H, Ikemoto S, Asaoka H, Hayakawa I, et al. Human macrophage scavenger receptors: primary structure, expression, and localization in atherosclerotic lesions. Proc Natl Acad Sci U S A. 1990; 87:9133–9137.



2. Ben J, Jin G, Zhang Y, Ma B, Bai H, Chen J, et al. Class A scavenger receptor deficiency exacerbates lung tumorigenesis by cultivating a procarcinogenic microenvironment in humans and mice. Am J Respir Crit Care Med. 2012; 186:763–772.


3. Caruso C, Balistreri CR, Candore G, Carruba G, Colonna-Romano G, Di Bona D, et al. Polymorphisms of pro-inflammatory genes and prostate cancer risk: a pharmacogenomic approach. Cancer Immunol Immunother. 2009; 58:1919–1933.


4. Krieger M, Abrams JM, Lux A, Steller H. Molecular flypaper, atherosclerosis, and host defense: structure and function of the macrophage scavenger receptor. Cold Spring Harb Symp Quant Biol. 1992; 57:605–609.


5. Krieger M, Acton S, Ashkenas J, Pearson A, Penman M, Resnick D. Molecular flypaper, host defense, and atherosclerosis. Structure, binding properties, and functions of macrophage scavenger receptors. J Biol Chem. 1993; 268:4569–4572.


6. Krieger M, Herz J. Structures and functions of multiligand lipoprotein receptors: macrophage scavenger receptors and LDL receptor-related protein (LRP). Annu Rev Biochem. 1994; 63:601–637.


7. Kodama T, Freeman M, Rohrer L, Zabrecky J, Matsudaira P, Krieger M. Type I macrophage scavenger receptor contains alpha-helical and collagen-like coiled coils. Nature. 1990; 343:531–535.


8. Krieger M. Molecular flypaper and atherosclerosis: structure of the macrophage scavenger receptor. Trends Biochem Sci. 1992; 17:141–146.


9. Yu X, Guo C, Fisher PB, Subjeck JR, Wang XY. Scavenger receptors: emerging roles in cancer biology and immunology. Adv Cancer Res. 2015; 128:309–364.


10. Facciponte JG, MacDonald IJ, Wang XY, Kim H, Manjili MH, Subjeck JR. Heat shock proteins and scavenger receptors: role in adaptive immune responses. Immunol Invest. 2005; 34:325–342.


11. Murshid A, Gong J, Stevenson MA, Calderwood SK. Heat shock proteins and cancer vaccines: developments in the past decade and chaperoning in the decade to come. Expert Rev Vaccines. 2011; 10:1553–1568.



12. Zhu H, Fang X, Zhang D, Wu W, Shao M, Wang L, et al. Membrane-bound heat shock proteins facilitate the uptake of dying cells and cross-presentation of cellular antigen. Apoptosis. 2016; 21:96–109.


13. Yu G, Tseng GC, Yu YP, Gavel T, Nelson J, Wells A, et al. CSR1 suppresses tumor growth and metastasis of prostate cancer. Am J Pathol. 2006; 168:597–607.



14. Luo HR, Liu Y, Wan XD, Li JL, Wu M, Zhang QM, et al. Sumoylation negatively regulates CSR1-dependent prostate cancer cell death. Cell Physiol Biochem. 2018; 46:1861–1867.


15. Bock AJ, Nymoen DA, Brenne K, Kærn J, Davidson B. SCARA3 mRNA is overexpressed in ovarian carcinoma compared with breast carcinoma effusions. Hum Pathol. 2012; 43:669–674.


16. Armengol C, Bartolí R, Sanjurjo L, Serra I, Amézaga N, Sala M, et al. Role of scavenger receptors in the pathophysiology of chronic liver diseases. Crit Rev Immunol. 2013; 33:57–96.


17. Liu H, Hu J, Pan H, Luo D, Huang M, Xu W. CSN5 promotes hepatocellular carcinoma progression by SCARA5 inhibition through suppressing β-catenin ubiquitination. Dig Dis Sci. 2018; 63:155–165.


18. You K, Su F, Liu L, Lv X, Zhang J, Zhang Y, et al. SCARA5 plays a critical role in the progression and metastasis of breast cancer by inactivating the ERK1/2, STAT3, and AKT signaling pathways. Mol Cell Biochem. 2017; 435:47–58.


19. Nakagawa-Toyama Y, Hirano K, Tsujii K, Nishida M, Miyagawa J, Sakai N, et al. Human scavenger receptor class B type I is expressed with cell-specific fashion in both initial and terminal site of reverse cholesterol transport. Atherosclerosis. 2005; 183:75–83.


20. Yang X, Sethi A, Yanek LR, Knapper C, Nordestgaard BG, Tybjærg-Hansen A, et al. SCARB1 gene variants are associated with the phenotype of combined high high-density lipoprotein cholesterol and high lipoprotein (a). Circ Cardiovasc Genet. 2016; 9:408–418.


21. Cedó L, Reddy ST, Mato E, Blanco-Vaca F, Escolà-Gil JC. HDL and LDL: potential new players in breast cancer development. J Clin Med. 2019; 8:E853.

22. Wang HH, Garruti G, Liu M, Portincasa P, Wang DQ. Cholesterol and lipoprotein metabolism and atherosclerosis: recent advances in reverse cholesterol transport. Ann Hepatol. 2017; 16 Suppl 1:S27–S42.

23. Graf GA, Roswell KL, Smart EJ. 17beta-Estradiol promotes the up-regulation of SR-BII in HepG2 cells and in rat livers. J Lipid Res. 2001; 42:1444–1449.


24. Gillard BK, Bassett GR, Gotto AM Jr, Rosales C, Pownall HJ. Scavenger receptor B1 (SR-B1) profoundly excludes high density lipoprotein (HDL) apolipoprotein AII as it nibbles HDL-cholesteryl ester. J Biol Chem. 2017; 292:8864–8873.



25. Shen WJ, Asthana S, Kraemer FB, Azhar S. Scavenger receptor B type 1: expression, molecular regulation, and cholesterol transport function. J Lipid Res. 2018; 59:1114–1131.



26. Shen WJ, Azhar S, Kraemer FB. SR-B1: a unique multifunctional receptor for cholesterol influx and efflux. Annu Rev Physiol. 2018; 80:95–116.


27. Wang J, Li Y. CD36 tango in cancer: signaling pathways and functions. Theranostics. 2019; 9:4893–4908.



28. Hale JS, Li M, Sinyuk M, Jahnen-Dechent W, Lathia JD, Silverstein RL. Context dependent role of the CD36--thrombospondin--histidine-rich glycoprotein axis in tumor angiogenesis and growth. PLoS One. 2012; 7:e40033.
29. Woo MS, Yang J, Beltran C, Cho S. Cell surface CD36 protein in monocyte/macrophage contributes to phagocytosis during the resolution phase of ischemic stroke in mice. J Biol Chem. 2016; 291:23654–23661.



30. Oury C. CD36: linking lipids to the NLRP3 inflammasome, atherogenesis and atherothrombosis. Cell Mol Immunol. 2014; 11:8–10.


31. Lawler PR, Lawler J. Molecular basis for the regulation of angiogenesis by thrombospondin-1 and -2. Cold Spring Harb Perspect Med. 2012; 2:a006627.

32. Simantov R, Febbraio M, Silverstein RL. The antiangiogenic effect of thrombospondin-2 is mediated by CD36 and modulated by histidine-rich glycoprotein. Matrix Biol. 2005; 24:27–34.


33. Uray IP, Liang Y, Hyder SM. Estradiol down-regulates CD36 expression in human breast cancer cells. Cancer Lett. 2004; 207:101–107.


34. DeFilippis RA, Chang H, Dumont N, Rabban JT, Chen YY, Fontenay GV, et al. CD36 repression activates a multicellular stromal program shared by high mammographic density and tumor tissues. Cancer Discov. 2012; 2:826–839.



35. Song L, Lee C, Schindler C. Deletion of the murine scavenger receptor CD68. J Lipid Res. 2011; 52:1542–1550.



36. Jiang Z, Shih DM, Xia YR, Lusis AJ, de Beer FC, de Villiers WJ, et al. Structure, organization, and chromosomal mapping of the gene encoding macrosialin, a macrophage-restricted protein. Genomics. 1998; 50:199–205.


37. Yang L, Yang L, Dong C, Li L. The class D scavenger receptor CD68 contributes to mouse chronic liver injury. Immunol Res. 2018; 66:414–424.


38. Ni YH, Ding L, Huang XF, Dong YC, Hu QG, Hou YY. Microlocalization of CD68+ tumor-associated macrophages in tumor stroma correlated with poor clinical outcomes in oral squamous cell carcinoma patients. Tumour Biol. 2015; 36:5291–5298.


39. Jézéquel P, Campion L, Spyratos F, Loussouarn D, Campone M, Guérin-Charbonnel C, et al. Validation of tumor-associated macrophage ferritin light chain as a prognostic biomarker in nodenegative breast cancer tumors: a multicentric 2004 national PHRC study. Int J Cancer. 2012; 131:426–437.


40. Ni C, Yang L, Xu Q, Yuan H, Wang W, Xia W, et al. CD68- and CD163-positive tumor infiltrating macrophages in non-metastatic breast cancer: a retrospective study and meta-analysis. J Cancer. 2019; 10:4463–4472.



41. Meng Y, Beckett MA, Liang H, Mauceri HJ, van Rooijen N, Cohen KS, et al. Blockade of tumor necrosis factor alpha signaling in tumor-associated macrophages as a radiosensitizing strategy. Cancer Res. 2010; 70:1534–1543.


42. Balzan S, Lubrano V. LOX-1 receptor: a potential link in atherosclerosis and cancer. Life Sci. 2018; 198:79–86.


43. Joo H, Li D, Dullaers M, Kim TW, Duluc D, Upchurch K, et al. C-type lectin-like receptor LOX-1 promotes dendritic cell-mediated class-switched B cell responses. Immunity. 2014; 41:592–604.



44. Hirsch HA, Iliopoulos D, Joshi A, Zhang Y, Jaeger SA, Bulyk M, et al. A transcriptional signature and common gene networks link cancer with lipid metabolism and diverse human diseases. Cancer Cell. 2010; 17:348–361.



45. Khaidakov M, Mitra S, Kang BY, Wang X, Kadlubar S, Novelli G, et al. Oxidized LDL receptor 1 (OLR1) as a possible link between obesity, dyslipidemia and cancer. PLoS One. 2011; 6:e20277.

46. Kumari S, Achazi K, Dey P, Haag R, Dernedde J. Design and synthesis of PEG-Oligoglycerol sulfates as multivalent inhibitors for the scavenger receptor LOX-1. Biomacromolecules. 2019; 20:1157–1166.






 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download