Introduction
Toxicodendron vernicifluum (Stokes) F.A. Barkley, also called as Rhus verniciflua is a deciduous tree belonging to Anacardiaceae family. It is widely distributed in Asia including Korea and also known as a lacquer tree. All parts of this plant are also used in traditional medicines and exerted diverse biological activities including antioxidant, anticancer, anti-inflammatory and antimicrobial effects have been reported.1234
Plants contain various bioactive substances. These substances are synthesized through biosynthesis, and each plant synthesizes different materials with specific biosynthetic enzymes. This represents the component diversity of each plant, which leads to the diversity of diversity. Differential composition and biological activity depending on the part have been reported by comparative analysis of different parts of plants.5678 For example, tanshinones and phenolic acids are abundant in roots of Salvia miltiorrhiza, whereas flavonoids and triterpenes are abundant in stems and leaves. Our previous study showed the difference of chemical constituents of each part, such as bark, lignum, leaf and fruit using LC-MS-MS and PCA analysis, and this eventually led to differences in biological efficacy.9 However, most of the studies on T. vernicifluum have been done on barks and there is little study on other parts.101112 Leaves are consumed as food, but little has been studied about its ingredients and benefits. This encourage us to investigate about the leaf of T. vernicifluum for wide application. In this study, three phenolic compounds, including two new compounds, were isolated from the leaves of T. vernicifluum and their structure were identified using spectroscopic analyses. Antioxidant efficacy of isolated compounds were also evaluated.
Experimental
Plant materials
The leaves of T. vernicifluum were collected from the farm in Buyeo, Korea in June 2016. After identification by the herbarium of the College of Pharmacy, Chungbuk National University, voucher specimen (CBNU2016-RVF) was deposited in a specimen room of the herbarium. The dried leaves of T. vernicifluum (1.5 kg) were extracted twice with 90% MeOH. The extract was evaporated under reduced pressure, which yielded the methanol extract (310 g). The methanol extract was suspended in water and partitioned successively with n-hexane, CH2Cl2 and EtOAc.
Extraction and isolation
The EtOAc fraction (RVE, 4.5 g) was chromatographed on a silica gel column chromatography and eluted with the mixture of CH2Cl2-MeOH with increasing polarity to give fifteen subfractions (RVE1-RVE15). RVE13 was chromatographed on MPLC (RP-18) and eluted with MeOH-H2O (10:90 to 100:0 gradient) to obtain 13 fractions (RVE13A - RVE13M). Compounds 1 (4.6 mg), 2 (4.7 mg) and 3 (1.9 mg) were purified from RVE13A by Sephadex LH-20 column chromatography eluted with CH2Cl2-MeOH (1:1) and followed by semi-preparative HPLC (MeOH-H2O, 20:80).
Rhuseoyl A (1)
brown syrup; [α]25D +10.4 (c 0.1, MeOH); UV (MeOH) λmax 216, 326 nm; IRmax 3324, 1654 cm−1; 1H NMR (500 MHz, CD3OD) and 13C NMR (CD3OD, 100MHz), see Table 1; ESI-MS (positive mode) m/z 335 [M+Na]+; HR-ESI-MS (positive mode) m/z 335.0737 (calcd for C14H16O8Na 335.0743).
+10.4 (c 0.1, MeOH); UV (MeOH) λmax 216, 326 nm; IRmax 3324, 1654 cm−1; 1H NMR (500 MHz, CD3OD) and 13C NMR (CD3OD, 100MHz), see Table 1; ESI-MS (positive mode) m/z 335 [M+Na]+; HR-ESI-MS (positive mode) m/z 335.0737 (calcd for C14H16O8Na 335.0743).
 +10.4 (c 0.1, MeOH); UV (MeOH) λmax 216, 326 nm; IRmax 3324, 1654 cm−1; 1H NMR (500 MHz, CD3OD) and 13C NMR (CD3OD, 100MHz), see Table 1; ESI-MS (positive mode) m/z 335 [M+Na]+; HR-ESI-MS (positive mode) m/z 335.0737 (calcd for C14H16O8Na 335.0743).
+10.4 (c 0.1, MeOH); UV (MeOH) λmax 216, 326 nm; IRmax 3324, 1654 cm−1; 1H NMR (500 MHz, CD3OD) and 13C NMR (CD3OD, 100MHz), see Table 1; ESI-MS (positive mode) m/z 335 [M+Na]+; HR-ESI-MS (positive mode) m/z 335.0737 (calcd for C14H16O8Na 335.0743).Rhuseoyl B (2)
brown syrup; [α]25D +11.9° (c 0.06, MeOH); UV (MeOH) λmax 217, 325 nm; IRmax 3323, 1654 cm−1; 1H NMR (500 MHz, CD3OD) and 13C NMR (CD3OD, 100 MHz), see Table 1; ESI-MS (positive mode) m/z 335 [M+Na]+; HR-ESI-MS (positive mode) m/z 335.0733 (calcd for C14H16O8Na 335.0743).
+11.9° (c 0.06, MeOH); UV (MeOH) λmax 217, 325 nm; IRmax 3323, 1654 cm−1; 1H NMR (500 MHz, CD3OD) and 13C NMR (CD3OD, 100 MHz), see Table 1; ESI-MS (positive mode) m/z 335 [M+Na]+; HR-ESI-MS (positive mode) m/z 335.0733 (calcd for C14H16O8Na 335.0743).
 +11.9° (c 0.06, MeOH); UV (MeOH) λmax 217, 325 nm; IRmax 3323, 1654 cm−1; 1H NMR (500 MHz, CD3OD) and 13C NMR (CD3OD, 100 MHz), see Table 1; ESI-MS (positive mode) m/z 335 [M+Na]+; HR-ESI-MS (positive mode) m/z 335.0733 (calcd for C14H16O8Na 335.0743).
+11.9° (c 0.06, MeOH); UV (MeOH) λmax 217, 325 nm; IRmax 3323, 1654 cm−1; 1H NMR (500 MHz, CD3OD) and 13C NMR (CD3OD, 100 MHz), see Table 1; ESI-MS (positive mode) m/z 335 [M+Na]+; HR-ESI-MS (positive mode) m/z 335.0733 (calcd for C14H16O8Na 335.0743).Measurement of antioxidant activity
The antioxidant activity was evaluated by measuring the DPPH radical scavenging activity. Briefly, samples were mixed with freshly prepared DPPH solution. After shaking, the reaction mixtures were stand for 30 min at room temperature in dark places. The radical scavenging activity was determined by measuring the absorbance at 517 nm. The relative radical scavenging activity (%) was calculated as [1 − absorbance of solution with sample and DPPH / absorbance of solution with DPPH] × 100.
Result and Discussion
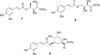
Investigation on the constituents of T. vernicifluum leaves yielded three compounds including two new compounds (Fig. 1).
Compound 1 was purified as brown syrup and its molecular formula was determined as C14H16O8 from the HRESI-MS (m/z 335.0737 [M+Na]+, calcd for C14H16NaO8, 335.0743) and 13C NMR data. The IR spectrum of 1 suggested the presence of hydroxyl (3324 cm−1) and carbonyl (1654 cm−1) functionalities. In the aromatic region of 1H NMR spectrum, signals attributed to 1,3,4,- trisubstituted aromatic ring and a double bond in trans-configuration were observed at [δH 7.06 (1H, d, J = 2.4 Hz, H-2′), 6.79 (1H, d, J = 8.0 Hz, H-5′), 6.97 (1H, dd, J = 8.0, 2.4 Hz, H-6′)] and [δH 7.61 (1H, d, J = 16.0 Hz, H-7′), 6.27 (1H, d, J = 16.0 Hz, H-8′)], respectively. The corresponding carbon signals at [δC 126.3 (C-1′), 113.7 (C-2′), 145.4 (C-3′), 148.3 (C-4′), 115.1 (C-5′), 121.7 (C-6′)] and [δC 146.2 (C-7′), 113.2 (C-8′)] in HSQC spectrum together with carbonyl signal at δC 166.8 (C-9′) clearly showed the presence of trans-caffeoyl moiety. Besides aforementioned signals, the 1H NMR spectrum revealed the presence of a methylene at δH 3.75 (1H, dd, J = 11.2, 6.8 Hz, H-1a), 3.83 (1H, dd, J = 11.2, 6.4 Hz, H-1b), two oxymethines at δH 5.30 (1H, ddd, J = 6.8, 6.4, 2.4 Hz, H-2) and δH 4.52 (1H, d, J = 2.4 Hz, H-3) and a methoxy group at δH 3.74 (3H, s, OCH3). The correlations between H-1 and H-2, H-2 and H-3 in 1H-1H COSY spectrum suggested the presence of -CH2-C(OH)-C(OH)- in 1. The corresponding carbon signals at δC 59.5 (C-1), 74.3 (C-2), 69.2 (C-3), 51.3 (OCH3), and carbonyl signal at δC 172.8 in the HSQC spectrum together with HMBC correlations of H-1 to C-2 and C-3, H-3 to C-4 and positive value of optical rotation suggested the presence of threonic acid.13 The HMBC correlations from δH 3.74 (3H, s, OCH3) to δC 172.8 (C-4) determined the position of a methoxy group (Fig. 2). The connection between a threonyl group and a caffeoyl moiety was assigned by the HMBC correlation from H-2 to C-9′, a carbonyl of caffeoyl moiety. Taken together, compound 1 was determined as shown in Fig. 1 and named rhuseoyl A.
Compound 2 was purified as brown syrup and same molecular formula of C14H16O8 was deduced from a pseudomolecular ion [M+Na]+ of m/z 335.0733 at the HRESI-MS analysis. Comparison of the 1H and 13C data of 2 with those of 1 disclosed that 2 also has trans-caffeoyl acid moiety. The presence of threonic acid was suggested by the signals at δH 4.27 (1H, brs, H-1a), 4.29 (1H, d, J = 6.4 Hz, H-1b), 4.23 (1H, m, H-2) and 4.32 (1H, d, J = 2.4 Hz, H-3), displaying 1H-1H COSY correlations between H-1 and H-2, H-2 and H-3 as in 1. Further examination of NMR data, however, disclosed the differences in the chemical shifts of H-1, H-2 and H-3. The chemical shift of H-1 was downfield shifted from δH 3.75 and 3.83 to δH 4.27 and 4.29, whereas that of H-2 was upfield shifted from δH 5.30 to δH 4.27 and 4.23. In addition, HMBC correlation from H-1 to C-9′ was observed in 2, instead of correlation from H-2 to C-9′ in 1 (Fig. 2). Therefore, 2 was suggested to differ from 1 in the linkage between caffeoyl acid and threonic acid as carbonyl of caffeoyl moiety was connected to C-1 of threonic acid. Taken together, compound 2 was determined as shown and named rhuseoyl B.
Compound 3 was identified as 5-O-(E)-caffeoylquinic acid methyl ester by the spectroscopic data analysis and comparison with those of published values.14
Evaluation of biological activity of isolated compounds suggested the DPPH radical scavenging effects of compounds 1 – 3 with IC50 values of 47.9, 107.8 and 15.4 µM, respectively. Taken together, our present study suggested that these compounds might contribute to the antioxidant properties of the leaves of T. vernicifluum, which will be useful for various oxidative stress mediated diseases.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


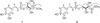

 XML Download
XML Download