Abstract
Luffa cylindrica (LC) is a very fast-growing climber and its fruit have been considered as agricultural wastes. We conducted to check the comparative qualities of ethanol solvent extraction (LCE) and supercritical carbon dioxide extraction (LCS) of L. cylindrica fruit and seed. LCS had higher antioxidant and polyphenol contents than LCE. LCS were significantly increased peroxisome proliferator-activated receptor (PPAR)-a and involucrin expression as epidermal differentiation marker in 3D skin equivalent model. LCS also showed antimicrobial activity against Staphylococcus aureus, a causative bacteria in atopic dermatitis. In addition, LCS inhibited the adipocyte differentiation of 3T3-L1 cells. When treated with the extract at a concentration of 100 µg/mL, the Wnt/β-catenin pathway reporter luciferase activity of HEK 293-TOP cells was increased approximately by 2-folds compared to that of the untreated control group. These results indicate that L. cylindrica supercritical carbon dioxide extract may serve as a cosmeceutical for improving skin barrier function and the treatment of obesity.
Medicinal plants contain natural substances that are considered traditional therapeutic agents. Numerous phytochemicals with potential biological activities in medicinal plants have been identified. The phytochemical materials found in medicinal plants belong to four major biochemical classes: alkaloids, glycosides, polyphenols, and terpenes.1
Despite profound therapeutic benefits of many biological compounds, most medicinal plants have been studied solely for the development of drugs and there are only a few reports on nutraceutical agents. Additionally, more effective and less toxic medicinal compounds are still required. In case the phenolic compounds are concerned, many different extraction techniques have been introduced, with the most frequent being with the use of water and organic solvents (e.g., ethanol, methanol).2 Ethanol, water and supercritical carbon dioxide are generally considered as safe solvents. The supercritical carbon dioxide (CO2) extraction method is an eco-friendly and has been successfully used to isolate the essential oil from natural products.3
The expression of PPAR-α promotes the synthesis of cholesterol and ceramides in keratinocytes during epidermal differentiation.2 In terms of keratinocyte differentiation and epidermal permeability barrier, PPAR-α agonists have been studied extensively, and it has been reported that treatment with PPAR ligands increases differentiation of the murine epidermis.4 Therefore, to determine whether the active agent of PPAR-α can change the rate of keratinocyte differentiation, a new agent must be found.
Wnt/β-catenin signaling is essential for adipogenesis and plays important physiological roles, in embryonic development and cellular proliferation.5 Many studies are underway to determine whether the Wnt/β-catenin inhibits adipogenesis.6 An understanding of molecular and cellular events regulating adipogenesis is important in designing rational treatments for prevention or treatment of obesity.7
Luffa cylindrica is a very fast growing climber, with oblong or cylindrical, smooth fruits containing many seeds which dry on the vine to develop into an inedible sponge-like structure. The seed of L. cylindrica have been considered agricultural wastes.8 Several studies have reported that the fruit of L. cylindrica shows positive biological effects, such as antioxidant9 and anti-inflammation effects.10 However, the effects of its fruit extract as a skin therapeutic agent for skin barrier function and as a nutraceutical product for obesity treatment have not been studied. In this study, we examined PPAR-α activity and adipogenesis activity with L. cylindrica fruit and seed extract. Here, we report that LC can be used in the development of cosmeceutical products for skin barrier dysfunction such as atopic dermatitis (AD) and obesity.
The L. cylindrica fruit and seed was purchased from the Jeonnam herbal farming cooperative (Hwasun-gun, Jeollanam-do, Korea). For supercritical co-solvent modified carbon dioxide extraction (LCS), the system and required components were acquired at 250 bar 50 ℃ 150 min at a flow rate of ethyl alcohol 3 mL/min for 90 min from Nano bio research center (Janseong-gun, Jeollanam-do, Korea). The extraction was performed by a method previously described.10 In case of LCE, L. cylindrica fruit and seed was soaked in 99.9% ethyl alcohol for 7 days and then was dried by speed vacuum at 50 ℃.
Cell viability was measured using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. Human normal keratinocytes were treated with different concentrations of LC for 48 h at 37 ℃, followed by the addition of 50 µL of 2 mg/mL MTT (Sigma-Aldrich, St. Louis, MO, USA) solution to respective wells, and then incubation for 3 h at 37 ℃. The procedure was conducted by the method previously described.13
PPAR-α transcription activity was performed using the PPAR response element (PPRE) transactivation method14 with slight modifications.
HEK 293-TOP cells (3 × 104) were seeded into 96-well plates and incubated in a medium with 10% FBS for one day. Total cell lysates were extracted with 25 µL 1x reporter lysis buffer (Promega, Madison, WI) per each well and luciferase activities were measured by adding 25 µL luciferin (USB, Cleveland, OH) per well using a microplate luminometer (BMG Labtech, Offenburg, Germany.11
The 3T3-L1 preadipocytes were cultured in Dulbecco's modified Eagle's medium (DMEM) with high glucose, 110 mg/L pyruvate supplemented with heat inactivated 10% (v/v) calf serum (Gibco, CA, USA), 100 µg/mL penicillin, 100 µg/mL streptomycin in a CO2 incubator at 37 ℃. To induce adipocyte differentiation, 3T3-L1 cells were cultured with DMEM plus 10% (v/v) heat inactivated fetal bovine serum (FBS) (Gibco) containing 520 µM isobutylmethylxanthine, 1 µM dexamethasone and 167 nM insulin. After 2 days, the media was then changed, and 167 µM of insulin in distilled water was added in a 1:1000 dilution. On day 4, medium was replaced with DMEM containing 10% FBS, and changed with fresh identical medium every 2 days. To measure the anti-differentiation effects of each drug, 3T3-L1 preadipocytes were induced to differentiate in the presence of different concentrations of LiCl, plant extracts. Dye solution was prepared as follows: 150 mg Oil Red O was dissolved in 30 mL of isopropanol. Then, the precipitate was removed by filtration and the supernatant was stored at room temperature after 20 mL of distilled water was added. Adipocyte cell layers were washed with PBS, fixed with 4% paraformaldehyde in PBS for 15 minutes at room temperature, stained with the Oil Red O dye solution for 1 h, and then washed with distilled water. Cells were checked by bright-field optical microscope (Nikon TE-200U, Tokyo, Japan).11
Skin models were prepared and cultured as described in Materials and methods and the experiments were performed by methods previously described.12 The effect of LCS on involucrin synthesis was analyzed using EpiDermTM, a three-dimensional model of skin equivalents purchased from MatTek Corporation (Ashland, MA, USA). The skin equivalents were stabilized in EPI-100-New maintenance medium (NMM, MatTek Co. Ashland, MA, USA) for 24 h and cultured in DMEM (Gibco-BRL/Life Technologies, Grand Island, NY, USA). After 12 days of culture, mature skin equivalents were harvested for immunohistochemical studies. The skin fragments were embedded in paraffin, and placed at 60 ℃ for 1 h. The skin fragments were exposed twice with xylene for 10 min, twice with 100% ethanol for 5 min, and twice with 70% ethanol for 5min, followed by incubation for 10 min with Tris-buffered saline (TBS). After incubation, the fragments were placed with 3% H2O2 for 30 min and then washed three times with TBS. The skin fragments were stained with rabbit anti-involucrin antibody (Santa Cruz Biotechnology, Carlsbad, CA, USA) for 1 h at 37 ℃. After reaction, the fragments were washed three times with TBS, followed by incubation for 1 h at room temperature with horseradish peroxidase (HRP)-conjugated secondary antibodies. After extensive washing, the fragments were placed in 3,3′-diaminobenzidine (DAB) buffer containing DAB chromogen (Dako, Glostrup, Denmark) for 2 min and then stopped in distilled water. The prepared samples were observed by microscope (Carl Zeiss Inc., Oberkochen, Germany). The area was analyzed by involucrin with image quantitative analysis software (NIH ImageJ, version 1.61.).
The 2,2-diphenyl-1-picrylhydrazyl (DPPH) assay was performed to determine the antioxidant capacity of LCE and LCS. The IC50 (concentration required to obtain a 50% antioxidant effect), a commonly used parameter to indicate the antioxidant capacity, was also measured.15
Total phenolic content and other oxidation substrates were examined using the Folin Ciocalteau (FC) reagent. We used a previously described method.16 The FC assay relies on the transfer of electrons in alkaline medium from phenolic compounds to phosphomolybdic/phosphotungstic acid complexes, which are determined at 765 nm as gallic acid equivalents (GAE).
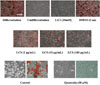
Staphylococcus aureus was used for paper disc diffusion assay and were first grown in Nutrient broth for 18 hrs at 37 ℃. After the top agar containing 5 × 105 CFU/mL hardened, dissolved samples 15 µL aliquot were dropped onto the surface of paper disc and incubated overnight at 37 ℃. If the sample examined had anti-microbial activity, a clear zone would form around paper disc, representing inhibition of strains growth.
All data are presented as the mean ± standard error of the mean (S.E.M). Statistical analyses were conducted with GraphPad Prism 5.0 (GraphPad Software, Inc. San Diego, CA, USA). Comparisons between multiple groups were performed using the one-way analysis of variance with Bonferroni posthoc test. P-value of less than 0.05 was considered significant.
The cell viability of human keratinocyte was not affected by 50 µg/mL of LCE and LCS (Fig. 1). The DPPH radical scavenging activities increased in a concentration-dependent manner. The SC50 of DPPH radical scavenging activity on LCE was 360.6 µg/mL and that of LCS was 349.1 µg/mL, that is, LCS has better antioxidant activity. This seems to be due to the higher polyphenol content of LCS (Fig. 2A, 2B). In Fig. 3, LCE and LCS significantly showed PPAR-α ligand binding activity at 50 µg/mL with compared to untreated control group. These results show that L. cylindrica extract has the potential as a novel PPAR-α agonist. We previously showed that flavonoids, such as quercetin and isorhamnetin, influenced PPAR-α ligand binding activity.12 Therefore, other secondary metabolites of L. cylindrica may serve as potential compounds for improving skin barrier function.
The two extracts of L. cylindrica do not show significant differences, but in the overall data, LCS have lower cytotoxicity and have better effects. Supercritical carbon dioxide extraction is also a better way to obtain lipophilic substances that are easy to penetrate the skin. In addition, supercritical carbon dioxide extraction is an efficient and eco-friendly method for industrial mass production without using organic solvent.3 Finally, skin barrier homeostasis and anti-obesity effect were performed on LCS for further tests.
Involucrin is an essential cornified envelope component that can be used as biomarker for screening of new candidates for the improvement skin barrier function.17 Immunohistochemical analysis of involucrin related to keratinocyte differentiation was determined in 3D skin equivalent model. As shown in Fig. 4, the treatment by LCS (50 µg/mL) showed a significant increase of involucrin expression compared with treatment by 0.2% DMSO as a negative control in skin equivalents, in addition, the group treated with LCS showed higher level in the expression of involucrin compared to the group treated with the same concentration of WY14643. In the previous paper,10 LCS has been shown to be consistent with the increase involucrin in both human normal keratinocyte and 3D skin equivalents (Fig. 5).
Staphylococcus aureus commonly colonizes the skin of atopic dermatitis (AD) patients and contributes to the exacerbation of AD. colonization of AD skin by S. aureus exacerbates AD and may contribute to microbial dysbiosis, allergen sensitization, Th2/Th17 polarization, development of atopic march and food allergy in AD patients.18 LC extracts had the similar antimicrobial activity against S. aureus as methylparaben (MP), which seems to help improve atopic dermatitis by stratum corneum homeostasis.
In addition, we investigated whether LCS can regulate the Wnt/β-catenin signaling and adipogenesis. The relative luciferase activity was increased approximately by 2-folds with the treatment of LCS at concentrations of 100 µg/mL, compared to that of the non-treated control group (Fig. 6). Additionally, LCS inhibited adipocyte differentiation of 3T3-L1 cells in a concentration dependent manner (Fig. 7). These results indicate that LCS inhibits differentiation of 3T3-L1 cells, showing the role of the Wnt/β-catenin activator in the adipocyte. Wnt/β-catenin signaling has been known to modulate additional developmental processes of adipocytes and to play a role in the suppression of differentiation of pre-adipocyte cells of 3T3-L1 cells.6 Therefore, the Wnt/β-catenin pathway could be an effective target to treat obesity.19
Herbal plant extracts and their active phytochemical compounds have many biological properties. There were evidences on the anti-obesity effects of flavonoids, such as isoquercitrin,11 isorhamnetin,11 and quercetin,2021 and silibinin and polyphenol, such as epigallocatechin-3-gallate, genestin, resveratrol, curcumin,22 isoflavone,23 and baicalin. However, the flavonoids such as quercetin, isoquercitrin, and isorhamnetin were not correctly detected in LCS, presuming that the efficacy of LCS is due to other components (Data not shown). At present, there are several reports about the interaction between PPAR-α and Wnt/β-catenin signaling. PPAR-α activation, with its agonist clofibrate, inhibits Wnt/β-catenin signaling in renal fibrosis and pancreatic cancer cells.24 Conversely, Wnt/β-catenin activation promotes PPAR-α expression in Alzheimer disease.25 Overall, our results indicate that LCS activates PPAR-α for the strength of skin barrier function and inhibits adipocyte differentiation in relation to the Wnt/β-catenin signaling pathway for anti-obesity effect. This study suggests that LC have potential as cosmeceutical agents for improving skin barrier functions for atopic dermatitis and the treatment of obesity.
Figures and Tables
Fig. 1
Effect of LC on cell viability. Ethanol solvent extraction and supercritical carbon dioxide extraction from L. cylindrica indicate LCE and LCS respectively. No treatment was used as a negative control. Values are presented as means ± standard error of the mean (SEM). *P < 0.05 compared to the untreated control group.

Fig. 2
(A) Antioxidant effect on LC. LC indicate Luffa cylindrica extract. (B) Total polyphenol content of LC. GAE indicates gallic acid equivalent in mg/g. Values are presented as means ± standard error of the mean (SEM).

Fig. 3
Transactivation of peroxisome proliferator-activated receptor (PPAR)-responsive element (PPRE) by different concentrations of LC. HEK293 cells were transfected with PPRE luciferase construct. WY14643 was used as a positive control and no treatment was used as a negative control. Values are presented as means ± standard error of the mean (SEM). *P < 0.05, compared to the untreated control group. LC indicate Luffa cylindrica extract.

Fig. 4
Immunoblot analysis of involucrin in 3D skin equivalent model. Each signal was quantified by ImageJ. Values are presented as means ± standard error of the mean (SEM). *P < 0.05 compared to the untreated control group. LCS indicate supercritical carbon dioxide extraction from L. cylindrica.

Fig. 5
Antimicrobial effect. S. aureus were used for paper disc diffusion assay. 10% DMSO was used as a negative control. Methyl paraben (MP) was used as a positive control. LC indicate Luffa cylindrica extract. A clear zone was examined around the paper disc. **P < 0.01 compared to the control group.

Fig. 6
Effect of LCS on luciferase activity. HEK293 cells containing pTOPFlash reporter gene in its chromosome were cultured and treated with different LC at 1, 10 or 100 µg/mL concentration, and cellular extract was prepared after 24 h as described in the Materials and methods. LCS indicate supercritical carbon dioxide extraction from L. cylindrica.

Fig. 7
Effect of LCS on the adipocyte differentiation of 3T3-L1 preadipocyte cells. 3T3-L1 cells were induced to differentiation for 7 days with or without 20 mM LiCl. Intracellular lipids of 3T2-L1 cells were visualized by Oil Red O staining as described in the Materials and methods. Original magnification: ×400. LCS indicate supercritical carbon dioxide extraction from L. cylindrica.
Acknowledgements
The author is grateful to Enprani Co. Ltd and Translational Research Center for Protein Function Control in Yonsei University for their support of this project and technical assistance.
References
1. Altemimi A, Lakhssassi N, Baharlouei A, Watson DG, Lightfoot DA. Plants. 2017; 6:42–65.
2. Rivier M, Safonova I, Lebrun P, Griffiths CE, Ailhaud G, Michel S. J Invest Dermatol. 1998; 111:1116–1121.
3. Tyśkiewicz K, Konkol M, Roj E. Molecules. 2018; 23:2625–2652.
4. Komuves LG, Hanley K, Lefebvre AM, Man MQ, Ng DC, Bikle DD, Williams ML, Elias PM, Auwerx J, Feingold KR. J Invest Dermatol. 2000; 115:353–360.
5. Liu J, Farmer SR. J Biol Chem. 2004; 279:45020–45027.
6. Kennell JA, MacDougald OA. J Biol Chem. 2005; 280:24004–24010.
7. Fruhbeck G, Gomez-Ambrosi J, Muruzabal FJ, Burrell MA. Am J Physiol Endocrinol Metab. 2001; 280:E827–E827.
8. Heiser CB, Schilling EE. Biotropica. 1988; 20:185–191.
9. Du Q, Xu Y, Li L, Zhao Y, Jerz G, Winterhalter P. J Agri Food Chem. 2006; 54:4186–4190.
10. Kim B, Lee SM, Hwang T, Kim H. Korean J Food Preserv. 2013; 20:597–601.
11. Lee SH, Kim B, Oh MJ, Yoon J, Kim HY, Lee KJ, Lee JD, Choi KY. Phytother Res. 2011; 25:1629–1635.
12. Kim B, Choi YE, Kim HS. Phytother Res. 2014; 28:1359–1366.
13. Kim B, Kim HS. Eur J Pharmacol. 2018; 832:25–32.
14. Kim SH, Nam GW, Lee HK, Moon SJ, Chang IS. Arch Dermatol Res. 2006; 298:273–282.
15. Sharma KV, Sisodia R. J Radiol Prot. 2009; 29:429–443.
16. Dall'Acqua S, Cervellati R, Loi MC, Innocenti G. Food Chem. 2008; 106:745–749.
17. Jensen JM, Folster-Holst R, Baranowsky A, Schunck M, Winoto-Morbach S, Neumann C, Schutze S, Proksch E. J Invest Dermatol. 2004; 122:1423–1431.
18. Kim J, Kim BE, Ahn K, Leung DYM. Allergy Asthma Immunol Res. 2019; 11:593–603.
19. Christodoulides C, Lagathu C, Sethi JK, Vidal-Puig A. Trends Endocrinol Metab. 2009; 20:16–24.
20. Seo MJ, Lee YJ, Hwang JH, Kim KJ, Lee BY. J Nutr Biochem. 2015; 26:1308–1316.
21. Kim MH, Park JS, Seo MS, Jung JW, Lee YS, Kang KS. Cell Prolif. 2010; 43:594–605.
22. Ahn J, Lee H, Kim S, Ha T. Am J Physiol Cell Physiol. 2010; 298:C1510–C1516.
23. Kim MH, Park JS, Seo MS, Jung JW, Lee YS, Kang KS. Cell Prolif. 2010; 43:594–605.
24. Ahn J, Lee H, Kim S, Park J, Ha T. Biochem Biophys Res Commun. 2008; 373:545–549.
25. Xue J, Zhu W, Song J, Jiao Y, Luo J, Yu C, Zhou J, Wu J, Chen M, Ding WQ, Cao J, Zhang S. Oncogene. 2018; 37:953–962.
26. Vallee A, Lecarpentier Y. Front Neurosci. 2016; 10:459.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download