Abstract
Cuscuta chinensis Lam. and Cuscuta japonica Choisy are parasitic plants. C. chinensis seeds were traditionally used for treatment of kidney and liver deficiencies. C. japonica seeds were used as tonic medicine to improve liver function and strengthen kidneys, treatment of high blood pressure, chronic diarrhea, and sore eyes. Cuscutae Semen are seeds of only C. chinensis in Korean Herbal Pharmacopoeia (K.H.P.). The developed HPLC-PDA method easily, accurately, and sensitively quantified using eight marker compounds [hyperoside (1), astragalin, (2), quercetin (3), kaempferol (4), chlorogenic acid (5), 3,4-di-O-caffeoylquinic acid (6), 1,5-di-O-caffeoylquinic acid (7), and 4,5-di-O-caffeoylquinic acid (8)]. In addition, the method may be used to distinguish seeds between C. chinensis Lam. and C. japonica Choisy. Furthermore, the result from the current study was applied to clarify samples between steam processed and unprocessed samples of C. chinensis by pattern analysis.
Cuscuta chinensis Lam. is a parasitic plant belonging to the Convolvulaceae family. It is widely distributed in Korea, China, Japan, and Africa.1 Stems are yellow and thin. Seeds were pale brown, ovoid, scabrous, and diameter of 1 - 3 mm. It was traditionally used as a medicine for the treatment of kidney and liver deficiency.2 In addition, pastes of this plant are used to treat chronic ulcer, wounds, painful inflammation, sore head, and inflamed eyes.1 In Korea and Vietnam, seeds of C. chinensis are applied for sexual function, health, and back pain.3 Several studies reported that C. chinensis possesses various biological activities such as hepatoprotective, antiosteoporotic,4 neuroprotection,5 antioxidant,6 anti-aging,7 anti-cancer, and anti-diabetic activities.8 Chemical constituents of C. chinensis are reported as flavonoids, alkaloids, steroids, fatty acid, volatile oils, lignans, quinic acid derivatives and polysaccharides.9 Flavonoids, a large group in natural products, reveal various pharmacological activities. Some flavonoids in this plant such as kaempferol, hyperoside, astragalin, and quercetin relate to the mechanism of clinical effects. Therefore, main flavonoid components in this plant would be interesting in determining its quality evaluation.
C. japonica Choisy is a typical dodder with yellowish vines or purplish spots, lightly tout. Seeds were brown with ovoid capsule and 3 – 5 mm in diameter. They were used as tonic medicine to improve liver function and strengthen kidneys, treatment of high blood pressure, chronic diarrhea, and sore eyes. In the Korean herb market, there are confused uses of seeds of C. japonica as Cuscutae Semen because it is difficult to distinguish dried seed specimens between C. chinensis and C. japonica. However, Cuscutae Semen in K.H.P. are seeds of only C. chinensis. Different species, environmental conditions, and locations lead to differences in chemical constituents and pharmacological properties.10 Therefore, an effective characterization method for quality control of seeds between C. chinensis and C. japonica is desirable. Until now, there are few studies on quantitative constituents in C. chinensis as well as C. japonica. Therefore, in this study, we describe development of the analysis method to simultaneously determine eight marker compounds with simple, rapid, and accurate analysis by reversed phase liquid chromatography in C. chinensis and C. japonica samples.
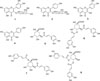
Unless specified, all reagents used were of analytical grade. The standards were purchased from Sigma-Adrich Chemical Co. (St. Louis, MO, USA). The standard compound structures are shown in Fig. 1. Acetonitrile (ACN) was purchased from Fisher Scientific Korea LtD. Water was purified using a Milli-Q system (Millipore, Bedford, USA).
The seeds of C. chinensis (C01 to C06) and C. japonica (J07 to J12) were identified and authenticated by Prof. Byung Sun Min. The voucher specimens (C.2017001 to C.2017012) of the samples were deposited in Herbarium at College of Pharmacy, Daegu Catholic University, Korea.
Standard stock solutions were prepared separately for each analytical standard and an internal standard (IS) in 2 mL MeOH at 1000 µg/mL and diluted with MeOH to obtain appropriate concentrations for content determination. The solutions were transferred to 10 mL amber glass vials, sealed using elastic plastic film (Parafilm, Chicago, IL, USA) and stored in a refrigerator (4℃) for analysis.
The dried seeds of C. chinensis was pulverized and passed through a 180 mesh sieve. About 1 g of the powder, accurately weighed, were added to 40 mL of 75% methanol containing an internal standard (IS: 20 ppm caffeic acid) by sonication for 60 minutes. After extraction, each sample solution was adjusted to the original volume and filtered through 0.45 µm membrane, and an aliquot (10 µL) of the filtrate was injected into HPLC.
The HPLC experiments were performed using a Waters Alliance system (Waters, Houston, TX, USA) equipped with a vacuum degasser, PDA detector and an Aegispak C18-L (250 mm × 4.6 mm, 5 µm) column. Data handling was managed by Empower v.3.0 software.
HPLC-PDA analyses were performed on a Waters (Houston, TX, USA) equipped with an autosampler, degasser, quaternary solvent pump, and PDA detector (Waters 2998) scanning in the wavelength range of 190 – 400 nm. Separation was carried out on an Aegispak C18-L column (4.6 × 250 mm, 5 µm particle size; Young JinBiochrom, Korea). UV detection was recorded at the wavelengths of 349 nm. The mobile phase consisted of solvent A (H2O containing 0.3% formic acid) and solvent B (acetonitrile), and gradient elution profile was conducted as follows: 0 – 30 min, 17% B; 30 – 50 min, 17 – 55% B. The flow rate for mobile phase was set at 1 mL/min and the injection volumes were 10 µL.
The analytical method for the seeds of C. chinensis was validated by the determination of linearity, limit of detection (LOD) and limit of quantitation (LOQ), accuracy, precision, stability, and robustness.11
Eight marker compounds were accurately weighed and dissolved in methanol 1000 µg/mL as stock solutions. The stock solutions were then diluted to produce different concentrations for each marker. Linearity was determined by plotting the measurements of area peak ratios (analyte/IS) versus concentrations of analytical standards. The sensitivity was expressed by the LOD and LOQ. The LOD represents the lowest concentration that can be reliably determined at a signal-to-noise (S/N) ratio of 3. The estimate for the LOQ was calculated using S/N ratio of 10.
Intra-day (n = 5) and inter-day (n = 5) precisions and accuracies were evaluated by analyzing sets of five independent samples at the low, mid, and high concentration levels. The precision was expressed as RSD% and the accuracy was expressed as bias. The stability of marker compounds was analyzed by the sample solution of aerial parts of C. chinensis through storing extract solution in the dark at 4℃ and at room temperature (25℃). The two samples were analyzed in triplicate at 0, 1, 3, 7, 15, and 30 days separation.12
The determination of the phytotaxonomic or phytochemical relationship of 12 samples [six C. chinensis Lam. (C01–C06) and six C. japonica Choisy (J07–J12)], pattern recognition analysis was conducted using software IBM SPSS Statistics Version 22.
A gradient RP-C18 HPLC system was performed for the simultaneous quantitative determination of eight compounds: hyperoside (1), astragalin, (2), quercetin (3), kaempferol (4), chlorogenic acid (5), 3,4-di-O-caffeoylquinic acid (6), 1,5-di-O-caffeoylquinic acid (7), and 4,5-di-O-caffeoylquinic acid (8) in extracts from C. chinensis and C. japonica seeds. These compounds showed a high UV absorption at 360 nm, therefore this wavelength was used for their quantitative determination. Mixtures of ACN (B) and water containing 0.3% formic acid (A) were examined as the mobile phases, with different ratios as well as a gradient elution system were optimized. The most suitable gradient elution system was 0 – 30 min, 17% (B); 30 – 50 min, 17 – 55% (B), v/v. The chromatographic peaks of the markers in the sample solution were determined by comparing their retention times with those of the individual standards, and were confirmed by spiking the samples with the individual compounds. Compounds (1 – 4) appeared in the chromatograms of C. chinensis samples, meanwhile compound (5 – 8) appeared in those of C. japonica samples (Fig. 2). The resolutions of all marker compounds in this chromatogram are much better and clearer than the previous report.13
Extraction process was optimized by using methanol-water and ethanol-water with different ratios. In this study, the seeds of C. chinensis and C. japonica were grinded for 5 min and were then sieved through a 250 µm2 sieve.12 The various solvent systems of ethanol-water mixtures (95%, 75%, 50%, 25%) and methanol-water (100%, 75%, 50%, 25%) were used to analysis of the C. chinensis and C. japonica samples to maximize recovery of all markers. The C. chinensis and C. japonica samples (each sample, 1.0 g) were extracted with 40 mL of above solvents for 60 min at room temperature in an ultrasonic bath. As a result, 75% aqueous methanol was selected as extract solvent system for C. chinensis and C. japonica samples due to the greatest peak areas of the markers 1 – 4 and 5 – 8 in their extraction, respectively. Similarly, the ultra-sonication was selected due to their higher area peaks in ultra-sonication than those of the reflux. To evaluate the optimized extraction times, five period times (30, 45, 60, 75, and 90 min) were examined in 75% methanol via sonication at room temperature. The results showed that each area peak of analytes was not increased from 60 min. Therefore, the extraction time was optimized as 60 min.
The linearity was evaluated by using seven different concentrations of each analyte (0.625, 6.25, 12.5, 25, 50, 100, and 200 µg/mL) for C. chinensis sample and (0.625, 6.25, 12.5, 25, 50, 100, 200, and 500 µg/mL) for C. japonica sample and in triplicate analysis. The calibration curves of each analyte showed excellent linearity over the tested range (r2 > 0.9991) (Table 1). The LOD of each analyte was determined to be 0.006 to 0.086 µg/mL and the LOQ was 0.023 to 0.288 µg/mL indicating that the developed method for the seeds of C. chinensis and C. japonica exhibited good sensitivity for determination of these marker compounds. The accuracy of the developed HPLC method was determined by analyzing the known amounts at the three different concentrations (each analyte: 1, 50, and 200 µg/mL) of analytes spiked into 75% methanol extract solution of the seeds of C. chinensis (marker compounds: 1 – 4) and C. japonica (marker compounds: 5 – 8). After addition of known amounts of each analyte to the previous 75% methanol extract solution, recovery studies were carried out. The results were listed in Table 2. In C. chinensis and C. japonica samples, the precisions for marker compounds (1 – 8) were less than 12.61% in intra-day and 13.25% in inter-day. The accuracies of the method were in the range 87.82 – 107.48% in intra-day and 87.02 – 111.13% in inter-day. With above data, the method developed was precise, accurate, and reliable for quantitation analysis of the two species (C. chinensis and C. japonica).
To evaluate the stability of the analytes, the 75% methanol extract solution of C. chinensis and C. japonica samples at room temperature (25℃) and 4℃ were measured at 0, 1, 3, 7, 15, and 30 days. As the results, the marker compounds (1 – 8) showed stable with recovery ranging from 98.1 to 101.6% (Table 3).
The contents of each analyte present in each sample (C. chinensis and C. japonica) were listed in Table 4, in which C01 - C03 samples of C. chinensis samples were steam processed. Two main analytes, compounds 1 and 4, were found in C. chinensis samples with the average values of 0.0138 and 0.039% on dry weight basis, respectively. Meanwhile, compounds 5 – 8 were not shown in their chromatogram (Fig. 2). The peak of compound 3 appeared in the chromatogram of steam processed samples C01 – C03. But, it was not detected in that of not-steamed samples C04 – C06. The contents of hyperoside in steam processed C. chinensis samples C01 – C03 (0.0153 – 0.0295%) were much greater than those of unprocessed samples C04 and C05 (0.0029 and 0.0065%) (Table 4).
A gradient RP-C18 HPLC system was conducted for simultaneous quantitative determination of eight compounds: hyperoside (1), astragalin, (2), quercetin (3), kaempferol (4), chlorogenic acid (5), 3,4-di-O-caffeoylquinic acid (6), 1,5-di-O-caffeoylquinic acid (7), and 4,5-di-O-caffeoylquinic acid (8) in extracts from C. chinensis and C. japonica seeds. Contents of each analyte present in each sample (C. chinensis and C. japonica) were listed in Table S5. Samples C01 – C03 from C. chinensis were steam processed. Two main analytes, compounds 1 and 4, were in C. chinensis samples with average values of 0.0138 and 0.039% on dry weight basis, respectively. Meanwhile, compounds 5 – 8 were not in their chromatogram. Content of hyperoside in steamed C. chinensis samples C01 – C03 (0.0153–0.0295%) was much greater than those of conventional samples C04 and C05 (0.0029 – 0.0065%). A gradient RP-C18 HPLC system was performed for the simultaneous quantitative determination of eight compounds: hyperoside (1), astragalin, (2), quercetin (3), kaempferol (4), chlorogenic acid (5), 3,4-di-O-caffeoylquinic acid (6), 1,5-di-O-caffeoylquinic acid (7), and 4,5-di-O-caffeoylquinic acid (8) in the extracts from C. chinensis and C. japonica seeds.
The contents of each analyte present in each sample (C. chinensis and C. japonica) were listed in Table 4. Samples C01 – C03 from C. chinensis were steam processed. Two main analytes, compounds 1 and 4, were found in C. chinensis samples with the average values of 0.0138 and 0.039% on dry weight basis, respectively. Meanwhile, compounds 5 – 8 were not shown in their chromatogram (Fig. 2). The content of hyperoside in steam processed samples of C. chinensis C01 – C03 (0.0153 - 0.0295%) were much greater than those of unprocessed samples C04 and C05 (0.0029 and 0.0065%) (Table 4). In C. japonica samples, compounds 5 and 7 were major constituents with average values of 0.3274 and 0.2563% (w/w), respectively. Interestingly, compounds 1 – 4 were not revealed in their chromatogram. Therefore, this developed method may be easily and accurately used to distinguish between C. chinensis and C. japonica. Cluster analysis of 12 samples was conducted on SPSS software using contents of seven marker components [hyperoside (1), astragalin, (2), quercetin (3), kaempferol (4), chlorogenic acid (5), 3,4-di-O-caffeoylquinic acid (6), 1,5-di-O-caffeoylquinic acid (7), 4,5-di-O-caffeoylquinic acid (8)]. Two species of C. chienensis Lam. and C. japonica Choisy were unambiguously distinguished by two different groups. In addition, two clusters of C. chinensis steam processed (C01–C03) and unprocessed samples (C04–C06) were also separated by the dendrogram (Fig. 3).
In conclusion, eight marker compounds (1 – 8) were identified in the C. chinensis and C. japonica samples. Particularly, development of the reliable HPLC-PDA method simultaneously quantitate eight marker compounds [hyperoside (1), astragalin, (2), quercetin (3), kaempferol (4), chlorogenic acid (5), 3,4-di-O-caffeoylquinic acid (6), 1,5-di-O-caffeoylquinic acid (7), 4,5-di-O-caffeoylquinic acid luteolin (8), an internal standard (caffeic acid). Results indicated that the method was applied for the quality evaluation of seeds between the C. chinensis and C. japonica.
Figures and Tables
 | Fig. 2The HPLC chromatograms of C. chinensis (A) and C. japonica (B) samples and the standard mixture (C). Hyperoside (1), astragalin (2), quercetin (3), kaempferol (4), chlorogenic acid (5), 3,4-di-O-caffeoylquinic acid (6), 1,5-di-O-caffeoylquinic acid (7), 4,5-di-O-caffeoylquinic acid (8), and an internal standard (IS, caffeic acid). |
 | Fig. 3The cluster analysis of C. chinensis and C. japonica seed samples using the contents of eight marker compounds (1 – 8). C. japonica seed samples (J07 – J12, A), C. chinensis steam processed (C01 – C03, B) and C. chinensis unprocessed seed samples (C04 – C06, C). |
Acknowledgments
This work was supported by the sabbatical research grant from Daegu Catholic University in 2018.
References
1. Donnapee S, Li J, Yang X, Ge AH, Donkor PO, Gao XM, Chang YX. J Ethnopharmacol. 2014; 157:292–308.
2. He XH, Yang WZ, Meng AH, He WN, Guo DA, Ye M. J Asian Nat Prod Res. 2010; 12:934–939.
3. Sam HV, Baas P, Kebler PJA. Blumea. 2008; 53:569–601.
4. Zhen GH, Jiang B, Bao YM, Li DX, An LJ. J Chin Med Mat. 2006; 29:1051–1055.
6. Yen FL, Wu TH, Lin LT, Lin CC. J Ethnopharmacol. 2007; 111:123–128.
7. Yao CH, Tsai HM, Chen YS, Liu BS. J Biomed Mater Res B Appl Biomater. 2005; 75:277–288.
8. Chauhan A, Sharma PK, Srivastava P, Kumar N, Dudhe R. Pharm Lett. 2010; 2:369–387.
9. Cheng P, Shi J, Du P, Liu D, Cao X, Wen X. Academic Periodical of Farm Products Processing. 2013; 8:116–118.
10. Liao GI, Chen MY, Kuoh CS. Bot Bull Acad Sin. 2005; 46:75–81.
11. Araujo P. J Chromatogr B Analyt Technol Biomed Life Sci. 2009; 877:2224–2234.
12. Le DD, Nguyen DH, Zhao BT, Min BS, Song SW, Woo MH. Nat Prod Sci. 2019; 25:122–129.
13. Hajimehdipoor H, kondori BM, Amin GR, Adib N, Rastegar H, Shekarchi M. Daru. 2012; 20:57–63.




 PDF
PDF ePub
ePub Citation
Citation Print
Print






 XML Download
XML Download