Abstract
Artocarpus heterophyllus has been used as traditional medicine. This plant is one of the sources of flavonoid. Flavonoid compounds possessed a wide range of biological properties including anticancer. This study was performed to investigate the cytotoxic effect of flavonoids from A. heterophyllus on H460 and MCF-7 cell lines. The interaction of flavonoids and cisplatin against tested cancer cells was also evaluated. MTT assay was used to determine the cytotoxic effect of flavonoid. Isobologram analysis was selected to evaluate the synergistic effect between flavonoid and cisplatin, their interaction was then confirmed using AO/PI staining method. Amongst of flavonoid compounds, artocarpin exhibited strong cytotoxic effect on both MCF-7 and H460 cell lines with IC50 values of 12.53 µg/mL (28.73 µM) and 9.77 µg/mL (22.40 µM), respectively. This compound enhanced anticancer activity of cisplatin against H460 and MCF-7. The combination produced a synergistic effect on H460 and MCF-7 cell lines with a combination index (CI) values of 0.2 and 0.18, respectively. The AO/PI stained demonstrated that the combination of artocarpin and cisplatin caused morphological changes that indicated apoptosis. Moreover, artocarpanone also significantly increased cytotoxic effect of cisplatin compared to its single concentration with CI below than 1. This result suggested the potency of flavonoid named artocarpin to enhance the anticancer activity of cisplatin on H460 and MCF-7 cell lines.
Lung cancer cells are the most common cancer in men, which is associated with tobacco smoking. It has been estimated that more than 1 million deaths per year all around the world due to this cancer.1 There are two broad histology types of lung cancer in which non-small cell lung cancer (NSCLC) accounts 85% of cases, while small cell lung cancer (SCLC) only causes 15%.2 On the other hand, breast cancer is still the most diagnosed cancer in women. Globally, it has been reported 1.3 million new cases with 0.5 million related deaths. This cancer can be identified according to the expression of specific cancer markers, such as oestrogen receptors (ER), Her2 oncogene, as well as progesterone receptor (PR).3 The use of chemotherapeutic agents and ionising radiation still become major options to eliminate tumour mass. There is evidence, however, that the combination of these therapies causes the incidence of tumor relapse that finally results in the development of drug resistance in cancer cells.4 Hence, it needs to find an alternative approach to reduce this limitation. Combination therapy using natural compounds as adjuvant in order to enhance the anticancer effect of commercial drug is one appealing strategy to overcome the resistance problem. The interaction between bioactive compounds and cytotoxic regiments may lead to the synergistic activity in targeted cancer cells.
In recent years, the use of herbal medicines based has become more popular and being accepted not only by local market, but also among western countries as it showed fewer side effects and safer compared to the modern treatment.5 Research studies have extensively focused on the antioxidant compounds of the natural product as it was revealed to possess antibacterial, anti-inflammatory, anticancer, antiviral, anti-aging and other effects.6 Artocarpus heterophyllus has been used as a traditional medicine to treat several illnesses.7 This plant is known contained various of flavonoid compounds.8 According to previous studies, various flavonoids, compounds such as artocarpanone (1), artocarpin (2), cycloartocarpin (3), and cyanomaclurin (4) (Fig. 1) have been successfully isolated from this plan.9 These compounds possessed several pharmacological properties such as antibacterial, anticariogenic antioxidant, immunomodulator, and anti-tyrosinase.1011121314 Although these compounds have been reported to have anticancer activity, however, there is no data available regarding their synergy effect in combination with commercial anticancer drugs. Therefore, the present study was undertaken to investigate the cytotoxic effect of flavonoid compounds as well as their synergistic effect when combined with commercial drugs.
Roswell Park Memorial Institute medium (RPMI-1640), Fetal Bovine Serum (FBS), Penicillin-Streptomycin, 3-(4,5-dimetylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT), and dimethylsulfoxide (DMSO), and cisplatin were purchased from Sigma Aldrich, St. Louis, MO. Artocarpanone, artocarpin, cycloartocarpin, and cyanomaclurin were isolated from Artocarpus heterophyllus heartwoods as previously described.7
Human breast cancer MCF-7 cell line and non-small lung cancer H460 cell line (ATC.HTB-177) was obtained from the American Type Culture Collection (ATCC) and cultured in complete medium containing RPMI-1640 media supplemented with 10% v/v fetal bovine serum, and 1% v/v penicillin-streptomycin in 25 cm2 T-Flask (GIBCO, USA). The cells were humidified atmosphere with 5% CO2 at 37℃.
Cytotoxic assay was performed according to Oladimenji et al. with slight modification.15 In brief, the cells (5 × 103 cells/well) were plated in a 96-well plate and incubated for 24 h. After incubation, five serial concentrations of compounds (31.25, 15.63, 7.81, 3.91, 1.95 µg/mL) were added into the plate and incubated for 72 h in 37℃ at 5% CO2. Cisplatin was used as positive control and untreated cell was used as a negative control. Next, 20 µL of thiazolyl blue tetrazolium bromide (MTT) reagent (5 mg/mL) is added into each well, after 4 h incubation, 150 µL of an absolute DMSO was added after aspiration of media. The cytotoxicity values were calculated after measuring the absorbance at 570 nm using a microplate reader. The experiment was repeated three times and the percentage of inhibition was calculated using the following formula:
The interaction of flavonoid compounds with cisplatin was determined using an isobologram analysis as previously described with slight modification.16 Briefly, the dose-dependent effects of each compounds and fixed concentrations of compound were determined using MTT assay. The cells were then treated with a combination of flavonoid compounds and cisplatin. The combination index (CI) was calculated to the analysis of synergy, antagonism, and additive effect using the following formula: In which Da1 is the concentration of flavonoid compound required to produce certain percentage when used alone, D1 is the concentration of flavonoid which has the same percentage in combination with cisplatin. While, Da2 is the concentration of cisplatin required to produce certain percentage when used alone, D2 is the concentration of cisplatin which has the same percentage in combination with flavonoid. The CI values were defined as synergy if CI < 1, additive if CI = 1, and antagonist if CI > 1.17
The microscopic evaluation of morphological changes and apoptosis features of cells treated with flavonoid compound and cisplatin were visualized using this method. The method was conducted according to Goh et al., with slight modifications.18 Briefly, H460 cells and MCF-7 cells were seeded and treated with respective IC50 values. After 72 hours of incubation with the treatments, the growth medium was discarded. The cells were then stained with the dye mixture containing 10 µL of 1 mg/mL acridine orange (AO) and 10 µL of 1 mg/mL of propopidium iodode (PI). The stained cells were examined and observed using inverted fluorescence microscope.
The cytotoxicity effect of flavonoid compounds against MCF-7 and H460 cell lines were determined using MTT assay. Amongst of testing compounds, artocarpin potentially induced highest cytotoxicity against both of MCF-7 and H460 cell line with IC50 values of 12.53 µg/mL (28.73 µM) and 9.77 µg/mL (22.40 µM), respectively (Table 1). The result was confirmed by observing the treated cells under microscope in which treatment with artocarpin caused cells damaged after 72 h incubation. In contrast, other compounds named cycloartocarpin, artocarpanone, cyanomaclurin, dyhidromorin only displayed mild cytotoxicity effect against MCF-7 and H460 cell lines.
Artocarpin seemed to be the most potent compound against both MCF-7 and H460 cell lines. It was in agreement with previous studies, artocarpin exhibited strong cytotoxic effect against human T47D breast cancer cells.19 Moreover, this result was also consistent with another previous finding associated with artocarpin in which not only induced ROS generation but also causing DNA oxidative damage in tumour cells.20 Correspondingly, there is extensive evidence that revealed prenyl group plays a crucial role in deciphering the associated cytotoxic properties in flavonoid compound. The investigation of the structure-cytotoxic activity on MCF-7 and H460 cell lines using the isolated compounds demonstrated that the isoprenyl flavones structures along its joined position and numbers of flavonoids improved their cytotoxicity.21
The use of chemotherapeutic agents for cancer treatment still remains challenging, in particular their resistance problem. Cisplatin is the most common chemotherapy drug that has been used for the treatment of various type cancers including breast and lung cancer. However, cancer gradually raises resistance to cisplatin through several mechanisms such as increased inactivation of drugs, reduced intracellular accumulation, and gain DNA repair.22 The alternative strategy to overcome this problem is the use of drug in combination. The interaction of two compounds in combination may enhance their activity. In case of synergy, different agents probably have different target of action and influence each site to achieve the same response. On the other hand, the different compounds may trigger the same site of action and it will generate agonistic activity.23
In this study, isobologram analysis was used to evaluate the interaction between flavonoid compounds and cisplatin. Artocarpin which exhibited strong cytotoxicity effect was selected to be combined with cisplatin in order to generate any synergistic effect against cancer cell lines. As displayed in Fig. 2, the IC50 value of artocarpin and cisplatin in combination was below the straight line. These results might indicate the synergistic interaction of flavonoid compound with cisplatin against H460 cells and MCF-7 cells. The CI was then analysed to verify the synergistic effect of flavonoid compound and cisplatin. Artocarpin in combination with cisplatin produced a synergistic effect in H460 cells and MCF-7 cells with CI values of 0.2 and 0.18, respectively.
The synergistic effect of artocarpin with cisplatin against H460 and MCF-7 cell lines were then confirmed using fluorescence microscope. Morphological observation of apoptosis was assessed with dual-fluorescence for live or dead nucleated cell concentration in heterogeneous samples using acridine orange (AO) and propidium iodide (PI). The orange AO and pink PI are the most used of nucleic acid binding dyes to measure the cell viability.242526 This AO/PI staining method was performed to observe viable, necrotic, or apoptosis cells after treatment with compound in combination. AO is a cell membrane permeable dye that only binds to DNA and RNA in live cells via intercalation meanwhile PI is a membrane impermeable molecule that is capable of binding to DNA and RNA upon the loss of integrity of cellular membrane in dead cells.27 Hoechst/PI or 7AAD are also another fluorescence-based dyes that can be used to measure viability, however, AO/PI was selected due to their stability and popularity for image-based viability analysis.
The result showed that the treatment with combination of artocarpin and cisplatin, the number of H460 and MCF-7 cell lines were lower than treatment with single compound. However, the number of apoptotic cells after treated with compound in combination were significantly higher compared to single compound (Fig. 3). The morphological observation in the cell nuclei of H460 and MCF-7 cells for 72 h after treatment with combination of artocarpin and cisplatin showed significant morphological changes compared to untreated control. In AO/PI image analysis, AO-stained live cells and PI stained dead cells in green and red fluorescence channels were observed. The viable cells displayed a uniform green fluorescence with the appearance of a circular cell with an intact nucleus. Early apoptotic cells have green nuclei with chromatin condensation and membrane blebbing while late apoptotic cells have orange to the red nucleus with condensed or fragmented chromatin. Necrotic cells display a uniform orange to red nucleus with a condensed structure.2829
The result has proved the potency of artocarpin, however, the interaction of this compound with commercial anticancer drugs; cisplatin needs to be explored more. Therefore, this study also focused on evaluation of synergistic effect of flavonoid compounds in enhancing the anticancer activity of cisplatin. In the present study, we found that artocarpin increase antitumor activity of cisplatin on H460 and MCF-7 cell lines. Furthermore, the combination of artocarpin and cisplatin increased the efficacy of each compound by producing a synergistic effect. This combination only required low concentration of each compound to achieve their cytotoxic effect on cancer cells. These results were then underlined using AO/PI staining method. It found that the cells treated with combination of artocarpin and cisplatin undergo certain morphological changes which indicated the features of apoptosis, including formation of DNA fragmentation, apoptotic bodies, early and late apoptosis, membrane cell blebbing, and chromatin condensation.
Interestingly, artocarpanone which had moderate activity on target cell also elevated activity of cisplatin on target cell lines. When used in combination, artocarpanone and cisplatin produced a synergistic effect. This combination also caused morphological changed on target cells and lead to apoptosis. These results indicated that a flavonoid compound may enhance cytotoxic effect of cisplatin to produce a synergistic effect. The finding was in agreement with previous studies, where it has been reported that deguelin, a flavonoid compound increase antitumor activity of cisplatin against gastric cancer cells.16 Furthermore, another study also reported that triptolide, a diterpene from Tripterygium wilfordii in combination with cisplatin produced a synergistic anticancer effect in gastric cancer cells. By this combination, triptolide increased apoptosis effect of cisplatin through activation of caspase-3 and caspase-9.30
In conclusion, artocarpin in combination with cisplatin displayed synergistic effects. These combinations produce significant anticancer activity in breast and non-small lung cancer cell lines. This finding suggests the potency of flavonoid compounds in enhancing anticancer activity of commercial drug named cisplatin. Nevertheless, further experiments are still required to elucidate their mechanism of action.
Figures and Tables
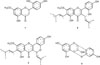 | Fig. 1Chemical structure of flavonoids isolated from A. heterophyllus heartwoods, artocarpanone (1), artocarpin (2), cycloartocarpin (3), and cyanomaclurin (4). |
 | Fig. 2Isobologram analysis of H460 (a) and MCF-7 (b) when treated with combination of artocarpin and cisplatin. |
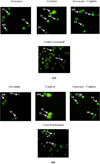 | Fig. 3Morphological changes of H460 (a) and MCF-7 (b) induced by cisplatin, artocarpin, and cisplatin-artocapin in combination. Arrows show (CI) Combination Index, (FL) Fluorescence, (AB) Apoptotic Body, (MB) Membrane Blebbing, (EA) Early Apoptosis, (LA) Late Apoptosis, (CC) Chromatin Condensation, (VI) Viable Cells, (DF) DNA Fragmentation. |
Acknowledgments
The authors would like to thank the Ministry of Education, Malaysia under the Fundamental Research Grant Scheme (FRGS 2017-2020) and Universiti Sultan Zainal Abidin (FRGS/1/2017/SKK06/UNISZA/03/1) for financial and technical supports.
References
1. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. CA Cancer J Clin. 2015; 65:87–108.
2. Oser MG, Niederst MJ, Sequist LV, Engelman JA. The Lancet Oncol. 2015; 16:e165–e172.
3. Tao Z, Shi A, Lu C, Song T, Zhang Z, Zhao J. Cell Biochem Biophys. 2015; 72:333–338.
4. Borghei YS, Hosseini M, Dadmehr M, Hosseinkhani S, Ganjali MR, Sheikhnejad R. Anal Chim Acta. 2016; 904:92–97.
5. Ahmad M, Khan MA, Marwat KS, Zafar M, Khan MA, Hassan TU, Sultana S. American-Eurasian J Agric Environ Sci. 2009; 5:126–140.
6. Cai Y, Luo Q, Sun M, Corke H. Life Sci. 2004; 74:2157–2184.
7. Salguero CP. A traditional recipes for health and harmony; Findhor. Scotland: Findhorn press;2003. p. 119.
8. Arung ET, Yoshikawa K, Shimizu K, Kondo R. Fitoterapia. 2010; 81:120–123.
9. Septama AW, Panichayupakaranant P. Pharm Biol. 2015; 53:1608–1613.
10. Septama AW, Panichayupakaranant P. Pharm Biol. 2016; 54:686–691.
11. Sato M, Fujiwara S, Tsuchiya H, Fujii T, Iinuma M, Tosa H, Ohkawa Y. J Ethnopharmacol. 1996; 54:171–176.
12. Septama AW, Jantan I, Panichayupakaranant P. J Pharm Pharmacol. 2018; 70:1242–1252.
13. Ko FN, Cheng ZJ, Lin CN, Teng CM. Free Radic Biol Med. 1998; 25:160–168.
14. Zheng ZP, Chen S, Wang S, Cheng KW, Wu JJ, Yang D, Wang M. . J Agric Food Chem. 2009; 57:6649–6655.
15. Oladimenji P, Cui H, Zhang C, Chen T. Expert Opin Drug Metab Toxicol. 2016; 12:997–1010.
16. Li P, Yang S, Dou M, Chen Y, Zhang J, Zhao X. J Cancer Res Clin Oncol. 2014; 140:2065–2075.
17. Zhang Z, Guo S, Liu X, Gao X. Drug Res. 2015; 65:214–218.
18. Goh SH, Mohamed Alitheen NB, Md Yussoff F, Yap SK, Loh SP. Pharmacogn Mag. 2014; 10:1–8.
19. Arung ET, Wicaksono BD, Handoko YA, Kusuma IW, Shimizu K, Yulia D, Sandra F. J Nat Med. 2010; 64:423–429.
20. Tsai MH, Liu JF, Chiang YC, Hu SC, Hsu LF, Lin YC, Lin ZC, Lee HC, Chen MC, Huang CL, Lee CW. Oncotarget. 2017; 8:28342–28358.
21. Chan EWC, Wong SK, Tangah J, Chan HT. Sys Rev Pharm. 2018; 9:58–63.
22. Amable L. Pharmacol Res. 2016; 106:27–36.
23. Yang Y, Zhang Z, Li S, Ye X, Li X, He K. Fitoterapia. 2014; 92:133–147.
24. Solomon M, Wofford J, Johnson C, Regan D, Creer MH. Transfusion. 2010; 50:820–830.
25. Chan LL, Wilkinson AR, Paradis BD, Lai N. J Fluoresc. 2012; 22:1301–1311.
26. Chan LL, Laverty DJ, Smith T, Nejad P, Hei H, Gandhi R, Kuksin D, Qiu J. J Immunol Methods. 2013; 388:25–32.
27. Chan LL, Kuksin D, Laverty DJ, Saldi S, Qiu J. Cytotechnology. 2015; 67:461–473.
28. Stankovic MS, Curcic MG, Zizic JB, Topuzovic MD, Solujic SR, Markovic SD. Int J Mol Sci. 2011; 12:4190–4205.
29. Alabsi AM, Ali R, Ali AM, Al-Dubai SA, Harun H, Abu Kasim NH, Alsalahi A. Asian Pac J Cancer Prev. 2002; 13:5131–5136.
30. Li CJ, Chu CY, Huang LH, Wang MH, Sheu LF, Yeh JI, Hsu HY. Cancer Lett. 2012; 319:203–213.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download