Abstract
A crude drug “Dang-Gui”, belonging to the genus Angelica, has been used as a traditional herbal medicine in Asia. Various studies have investigated the chemical components and pharmacological activities of Dang-Gui worldwide. However, domestic research results published in Korean are undervalued in international academia due to language barriers. Therefore, it is necessary to summarize the domestic research findings systematically for greater accessibility. This review focuses on the results published in four Korean pharmaceutical journals between 1970 and 2018, which detail the botanical, phytochemical, and pharmacological properties of three Angelica species (A. gigas, A. sinensis, and A. acutiloba) used as “Dang-Gui” in Korea, China, and Japan.
“Dang-Gui” refers to the roots of medicinal plants belonging to the genus Angelica (Umbelliferae) that have been widely used as traditional medicine throughout Korea, China, and Japan. Korean, Chinese, and Japanese Pharmacopoeia define Dang-Gui as a different botanical origin: Angelica gigas Nakai, Angelica sinensis (Oliv.) Diels and Angelica acutiloba Kitagawa, respectively.123 For example, in Korean Pharmacopoeia the roots of A. gigas are “Dang-Gui”, while the roots of A. acutiloba are “Il-Dang-Gui”, meaning Japanese Dang-Gui.
Multiple pharmaceutical studies on Dang-Gui have been conducted both domestically and worldwide. Between 1970 and 2018, approximately 400, 200, and 3600 international papers have been published about A. gigas, A. acutiloba, and A. sinensis, respectively. Unfortunately, due to language barriers, papers published in Korean journals are often disregarded. For this reason, domestic research results are underestimated in international academia. Therefore, it is necessary to summarize domestic research results systematically for international accessibility. This work summarizes domestic research performed on three Angelica species used as Dang-Gui in Korea, China, and Japan. This review is limited to studies published between 1970 and 2018 in four Korean pharmaceutical journals (Korean Journal of Pharmacognosy, Yakhak Hoeji, Natural Product Sciences, and Archives of Pharmacal Research), of which the former two are written in Korean. The number of relevant articles in these journals is 13, 5, 6 and 9, respectively, and their collective findings are summarized herein.
Comparative histological studies were carried out to clarify the origins of the three Angelica species.45 According to these studies, the three species are distinguished by the shape of the cork cortex, resin duct and xylem fiber, frequency and size of secretory cells, and the size of vessels. For example, A. sinensis can be recognized by the number of cork cells in the cork layer (4 – 7), which is the greatest among the three species. A. acutiloba has the largest resin duct diameter (200 – 300 µm) and the lowest duct frequency. In comparison, A. gigas displays the smallest duct diameter (20 – 60 µm) and the highest frequency among the three species. The diameter and frequency of the resin duct in A. sinensis are slightly larger than those of A. gigas. A. acutiloba also shows the greatest number (25 – 40) of secretory cells surrounding the resin duct, followed by A. sinensis and A. gigas (5 – 8). The xylem fibers of A. gigas are the most well–developed among the three species, while those of A. acutiloba and A. sinensis are similar to each other. A. acutiloba has the smallest vessel diameter (15 – 40 µm), while the vessel diameters of A. gigas and A. sinensis are similar (20 – 80 µm and 20 – 90 µm, respectively).
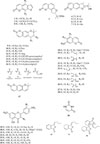
Dang-Gui has been shown to contain a variety of constituents including coumarins (1 – 28), flavonoids (29 – 35), and diverse essential oils (e.g., benzofuranone derivatives) (42, 74 and 82) (Fig. 1). The phytochemical components of Dang-Gui vary depending on the specific plant. Most domestic studies on Dang-Gui ingredients involved A. gigas because their roots are the primary source of this medicine in Korea.
The main components of A. gigas are decursin (10) and decursinol angelate (12), which are pyranocoumarins. Angelan is a pharmacologically active pectin polysaccharide isolated from A. gigas.6 The main component of A. acutiloba and A. sinensis is a benzofuranone, ligustilide (74), found in the essential oil fraction (Table 1).7 Most essential oils of the three Angelica species were identified by gas chromatography-mass spectrometry (GC-MS); however, further studies into the pharmacological activity and structure of butylidenephthalide (42), Z-ligustilide (74), and neodiligustilide (82) have been performed (Fig. 1).
Although the pharmacological activities of these three Angelica species have been reported in Korean and international journals, the results in Korean journals are difficult to find outside of Korea. Domestic research has shown that these Angelica species have anti-inflammatory, antibacterial, antioxidant, antihyperlipidemic, hepatoprotective, and neuroprotective activities (Table 2).
In addition to studies on pharmacological activity, drug metabolism studies have also been conducted on the components of the three Angelica species. Woo et al. used mouse hexobarbital-induced hypnosis to investigate the effects of methanol extracts of A. gigas and A. acutiloba on drug metabolism and found that the furanocoumarin components of the extracts affected the drug-metabolizing enzymes.29 Shin et al. showed that decursin (10), a major component of A. gigas, inhibited the hepatic enzyme system.30
To evaluate the efficacy of herbal medicines, Park et al. investigated drug interactions occurring during the administration of A. gigas extracts and other herbal medicines. In this study, they measured the concentration of decursinol (11) in blood, using decursin (10) and its metabolite decursinol (11) as indicator substances.20 This study found that oral administration of ether or methanol extracts of A. gigas resulted in higher concentrations of decursinol (11) in blood, as compared to treatment with decursin (10) alone. Coadministration of decursin (10) and Cnidii Rhizoma extracts increased the concentration of decursinol (11) in blood, while coadministration of decursin (10) and Bupleuri Radix extracts decreased the blood concentration of decursinol (11). However, coadministering Cnidii Rhizoma or Bupleuri Radix extracts with decursinol (11) increased the level of decursinol (11) in blood.
As most herbal medicine is generally distributed after cutting and drying, there is a limit to distinguish the origins and producing area only by histological discrimination. To address this limitation, a clearer method for discrimination has been developed. To establish such a method, Cho et al. used non-destructive analytical techniques, including near-infrared spectroscopy, X-ray fluorescence spectrometry, and electronic nose, to compare and analyze A. gigas and A. sinensis. All three methods showed a discrimination rate of 90% or higher. Additionally, these methods are fast and simple and require no preprocessing.34
Kim et al. compared the concentration of coumarins between roots of A. gigas cultivated in Korea and China. They found that marmesin (4), nodakenin (7), decursin (10), and decursinol (11) were higher on average in Korean than Chinese roots. They successfully distinguished Korean and Chinese roots of A. gigas using multivariate analysis [Principal Component Analysis (PCA), Partial Least Squares Discriminant Analysis (PLS-DA)], based on decursin (10) and decursinol angelate (12).35
Decursin (10) and decursinol angelate (12), which are major components of A. gigas, are structural isomers with similar chemical properties, making the two compounds difficult to isolate and purify. To overcome this limitation, the conditions for analytical reverse-phase high-performance liquid chromatography (HPLC) of decursin (10) and decursinol angelate (12) were explored.3637 The peaks were best separated using mobile phases composed of acetonitrile with sodium dodecyl sulfate and sodium dihydrogen phosphate, and acetonitrile with sodium lauryl sulfate and sodium phosphate. The optimum HPLC conditions were identified as a column temperature of 30 – 35℃, a flow rate of 1 – 1.2 ml/min, with UV detection at 230 or 280 nm.
An efficient, large-scale extraction process was proposed by comparing and analyzing the extraction efficiency of the components of A. gigas.36 Kang et al. reported that the concentrations of decursin (10) and decursinol angelate (11) in 100% ethanol extracts were slightly higher than those in 50% ethanol extracts. However, there were greater differences in the extraction efficiency between ethanol extracts and deionized water extraction. As there was not a significant difference between 100% and 50% ethanol extracts, it was predicted that the extraction process using 50% ethanol would be more suitable for safety engineering in large-scale extractions.
In addition, Lee et al. proposed a method for massproducing decursinol (11), a starting material for the synthesis of various derivatives, including decursin (10) and decursinol angelate (12), by hydrolyzing A. gigas extracts.14 This study established a method for obtaining pure target compounds solely through recrystallization following hydrolysis, without complicated separation processes. They succeeded in producing a large amount of decursinol (11) from the root of A. gigas, and found that the highest yield of decursinol (11) was obtained using NaOH. Additionally, they identified ether as the most effective solvent for hydrolysis.
Dang-Gui has been widely used as traditional medicine in Korea, China, and Japan and its botanical origins in the official compendia differ between the countries. The Korean pharmacopoeia defines the origin of Dang-Gui as A. gigas. In Korea, the most studied was carried out on A. gigas. Much less is known about the composition of A. sinensis than both A. gigas and A. acutiloba. Furthermore, there are no studies on the pharmacological activity of A. sinensis in Korea. Along with studies investigating the phytochemical components of the three Angelica species and the pharmacological activities of these components and extracts, this review showed four studies focused on classifying plant origin.
A variety of coumarins have been reported through studies investigating the components of Dang-Gui. Extracts and individual components of the three Angelica species were found to have anti-inflammatory, antibacterial, and antioxidant properties. Additionally, anticancer/cytotoxic, antihyperlipidemic, hypoglycemic, hepatoprotective, and neuroprotective activities of these extracts and individual compounds have been found to be effective in the prevention and treatment of lifestyle diseases such as diabetes, arteriosclerosis, and cancer. However, there are few studies on components other than coumarins, and studies relating to biological activity have focused primarily on decursin (10) and decursinol angelate (12), the main components of A. gigas and its extracts. Therefore, for a better understanding of Dang-Gui and its applications, it is required to expand the studies of various other compounds of this species and perform additional biological activity tests. In addition, continuous research on A. sinensis and A. acutiloba will improve the availability of Dang-gui for the modern medicinal uses.
Figures and Tables
References
1. The Korean Pharmacopoeia. 11th Ed. Republic of Korea: Ministry of Food and Drug Safety;2014. p. 1790.
2. The Japanese Pharmacopoeia. 16th Ed. Japan: The Ministry of Health Labor and Welfare;2012. p. 1866.
3. Pharmacopoeia of the People's Republic of China; National Pharmacopoeia Commission of the People's Republic of China. China: China Medical Science Press;2015. p. 133.
4. Park JH, Lee YJ, Keon SJ. Korean J Pharmacogn. 2005; 36:141–144.
5. Ahn MJ, Bae JY, Park JH. Korean J Pharmacogn. 2011; 42:103–106.
6. Kim HJ, Piao XL, Jang YP. Nat Prod Sci. 2011; 17:202–205.
7. Kim HM, Kang JS, Park SK, Lee K, Kim JY, Kim YJ, Hong JT, Kim Y, Han SB. Arch Pharm Res. 2008; 31:1489–1496.
8. Lee S, Shin DS, Kim JS, Oh KB, Kang SS. Arch Pharm Res. 2003; 26:449–452.
9. Lee S, Kang SS, Shin KH. Nat Prod Sci. 2002; 8:58–61.
10. Kang SY, Kim YC. Arch Pharm Res. 2007; 30:1368–1373.
11. Yook CS, Kim CW, Ryu KS. Korean J Pharmacogn. 1973; 4:189–190.
12. Lee YY, Lee S, Jin JL, Yun-Choi HS. Arch Pharm Res. 2003; 26:723–726.
13. Ryu KS, Hong ND, Kim NJ, Kong YY. Korean J Pharmacogn. 1990; 21:64–68.
14. Lee JH, Choi YS, Kim JS, Kim JH, Jeong HG, Kim DH, Yun MY, Kim JS, Cho SH, Shen GN, Kim EG, Jin WY, Song GY. Yakhak Hoeji. 2006; 50:172–176.
15. Moon HI, Ahn KT, Lee KR, Zee OP. Yakhak Hoeji. 2000; 44:119–127.
16. Lee S, Kang SS, Shin KH. Nat Prod Sci. 2002; 8:127–128.
17. Roh J, Lim H, Shin S. Nat Prod Sci. 2012; 18:244–249.
18. Chi HJ, Kim HS. Korean J Pharmacogn. 1988; 19:239–247.
19. Chen QC, Lee JP, Jin WY, Youn UJ, Kim HJ, Lee IS, Zhang XF, Song KS, Seong YH, Bae KH. Arch Pharm Res. 2007; 30:565–569.
20. Park RJ, Kim NJ, Lee KT, Seo SH. Korean J Pharmacogn. 2001; 32:72–78.
21. Son CY, Baek IH, Song GY, Kang JS, Kwon KI. Yakhak Hoeji. 2009; 53:303–313.
22. Kim NS, Jeong SI, Kim JS, Oh MJ, Oh CH. Korean J Pharmacogn. 2016; 47:197–203.
23. Lee S, Lee YS, Jung SH, Shin KH, Kim BK, Kang SS. Arch Pharm Res. 2003; 26:727–730.
24. Kim HM, Kang JS, Park SK, Lee K, Kim JY, Kim YJ, Hong JT, Kim Y, Han SB. Arch Pharm Res. 2008; 31:1489–1496.
25. Kim CM, Heo MY, Kim HP, Sin KS, Pachaly P. Arch Pharm Res. 1991; 14:87–92.
26. Kim YA, Park SH, Kim BY, Kim AH, Park BJ, Kim JJ. Korean J Pharmacogn. 2014; 45:209–213.
27. Lee S, Lee YS, Jung SH, Shin KH, Kim BK, Kang SS. Nat Prod Sci. 2003; 9:170–173.
28. Kim YK, Cho KH, Shon YH, Choi HK, Kim SY, Lim JK, Nam KS. Yakhak Hoeji. 2000; 44:283–292.
29. Woo WS, Shin KH, Ryu KS. Arch Pharm Res. 1980; 3:79–84.
30. Shin KH, Han JM, Lee IR. Korean J Pharmacogn. 1996; 27:323–327.
31. Chung MH, Oh HS, Lim JH. Korean J Pharmacogn. 1998; 29:402–412.
32. Chung MH, Lim JH, Oh HS. Korean J Pharmacogn. 1998; 29:300–311.
34. Cho CH, Kim SJ, Kim HJ. Yakhak Hoeji. 2002; 46:161–167.
35. Kim JR, Lee DY, Sung SH, Kim J. Korean J Pharmacogn. 2013; 44:332–335.
36. Kang YG, Lee JH, Chae HJ, Kim DH, Lee S, Park SY. Korean J Pharmacogn. 2003; 34:201–205.
37. Lee JP, Chang SY, Park SY. Nat Prod Sci. 2004; 10:262–267.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download