Abstract
Spontaneous intracranial hypotension (SIH) can be a rare risk factor of cerebral venous thrombosis. We describe a case of isolated cortical vein thrombosis (CVT) secondary to SIH and discuss the value of susceptibility-weighted imaging for the detection of isolated CVT.
Cerebral cortical vein thrombosis (CVT) can be presented as an isolated form or combined with venous sinus thrombosis (1). Isolated CVT is difficult to diagnose because there are many anatomical variations in the cerebral venous system (23). Susceptibility-weighted imaging (SWI) is a considerably sensitive MR sequence for the detection of intravascular deoxygenated blood as well as extravascular blood products. It has been reported SWI is valuable regarding the detection of intravascular thrombus(4).
An age 26 female was presented with a headache since two weeks ago. She complained of occipital headache, nausea, and vomiting when upright, which were completely relieved when lying down. Neurologic examination showed a mild reduction in motor power in both of her lower extremities. The cerebrospinal fluid (CSF) pressure was measured as 0 cm H2O on the lumbar puncture. Laboratory testing including CSF study was unremarkable. She denied recent history of trauma, lumbar puncture, acupuncture, hematologic disease, pregnancy, or oral contraceptive use.
CT and CT venography (7) were obtained in the emergency department. A cortical vein of the left cerebral vertex showed high attenuation on non-contrast image, which was not opacified on CT venography (Figs. 1, 2). On the second day of admission, brain MR examination was performed with a 3T scanner (Ingenia; Philips Healthcare, Best, the Netherlands). There was a small amount of subdural fluid collection. The pachymeninges were thickened and strongly enhanced after the administration of the Gadolinium contrast agent. There was a hyperintense lesion in a cortical vein of the left cerebral vertex on T1-weighted image, not detected on T2-weighted image. SWI showed a blooming artifact due to thrombus in and beyond the cortical vein (Fig. 1). MR myelography was performed on day four of hospitalization, but CSF leakage was not detected. Ten days after the initial CT venography, follow-up CT venography was obtained. The cortical vein thrombus showed iso-attenuation as the cerebral cortex on non-contrast image and remained a filling defect on CT venography (Fig. 2).
The patient received anticoagulation therapy from the second day of admission and her symptoms slowly improved. She was discharged a month later without neurological deficits.
Our patient showed typical clinical and MR findings of SIH such as orthostatic headache, low CSF pressure, subdural fluid collection, and diffuse pachymeningeal thickening (5). SIH is a rare risk factor of cerebral venous thrombosis. Approximately 2.1% of cases are complicated by venous thrombosis (6). The relationship between SIH and cerebral venous thrombosis has not been clearly established. SIH is commonly caused by CSF leakage into the spinal epidural space. The reduced intracranial CSF spaces are compensated by subdural fluid collection and enlargement of the cerebral venous spaces, leading to a reduction in the flow velocity of the cerebral venous system. Additionally, the loss of intracranial CSF volume may cause stretching of the cerebral venous system and damage to the venous lining. These changes facilitate the formation of thrombi in the cerebral cortical veins and venous sinuses (89).
CVT is usually associated with venous sinus thrombosis. SIH is a risk factor of cerebral venous sinus thrombosis, a certain case as in our patient may present as isolated CVT (1011). Thus, it is crucial to detect imaging evidence of isolated CVT as well as venous sinus thrombosis in the case of SIH. However, isolated CVT is rare and difficult to diagnose because there are many anatomical variations in the cerebral cortical veins and the CT attenuation and MR signal intensity of CVT change according to the age of thrombus (23) (Fig. 2). Even if digital subtraction angiography (DSA), CT venography or MR venography is performed, it is sometimes difficult to detect intravenous filling defects due to thrombi since the thrombosed cortical veins are not often opacified (Fig. 2b).
In this case, we could easily detect the isolated CVT with SWI (Fig. 1f). It has been reported that SWI is valuable regarding the detection of intravascular thrombus. In acute stroke patients with large vessel occlusion, SWI can detect a blooming artifact due to thrombus in and beyond the cerebral arterial lumen. Additionally, it can also be valuable in assessing the burden of thrombus, which may facilitate establishing a therapeutic strategy for thrombolysis (4). On SWI, the cerebral veins usually appear as dark signal intensity structures since they have relatively high content of deoxy-hemoglobin that produces a susceptibility effect (12). In our patient, the thrombosed cortical vein was significantly larger in diameter than the adjacent normal veins. Thus, it was not difficult to judge that it was caused by cortical vein thrombosis. The high signal intensity of the corresponding vein on T1-weighted image was also valuable regarding the diagnosis (Fig. 1d).
In summary, SIH can be a risk factor for isolated CVT. SWI is valuable regarding the diagnosis of isolated CVT.
Figures and Tables
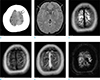 | Fig. 1An age 26 female with a headache. (a) The non-contrast CT scan shows a hyperdense cortical vein at the left cerebral vertex (arrows). (b) The axial fluid-attenuated inversion recovery image reveals small subdural fluid collection at both anterior cerebral convexities (arrows). (c) The T2-weighted image of the cerebral vertex shows no definite abnormality. (d) The T1-weighted image reveals a hyperintense lesion in the cortical vein of the left cerebral vertex (arrows). (e) On the contrast-enhanced T1-weighted image, the thrombosed cortical vein is not enhanced (arrows). (f) The susceptibility-weighted minimum intensity projection (minIP) image shows a blooming artifact due to thrombus in and beyond the cortical vein (arrows). |
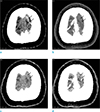 | Fig. 2An age 26 female with a headache. (a, b) The initial CT venography. (c, d) The follow-up CT venography, 10 days later. (a) The noncontrast CT scan shows a hyperdense cortical vein at the left cerebral vertex (arrows). (b) On the source image of the CT venography, the cortical vein is not opacified (arrows). (c) The follow-up non-contrast CT scan reveals the thrombosed cortical vein as iso-density to the cerebral cortex (arrows). (d) On the follow-up CT venography, there is a filling defect in the thrombosed cortical vein (arrows). |
Acknowledgments
This report was supported by the Development Fund Foundation, Gyeongsang National University, 2015.
References
1. Coutinho JM, Gerritsma JJ, Zuurbier SM, Stam J. Isolated cortical vein thrombosis: systematic review of case reports and case series. Stroke. 2014; 45:1836–1838.
2. Alvis-Miranda HR, Milena Castellar-Leones S, Alcala-Cerra G, Rafael Moscote-Salazar L. Cerebral sinus venous thrombosis. J Neurosci Rural Pract. 2013; 4:427–438.
3. Jang J, Choi DS, Shin HS, et al. Clinical and radiological manifestations of cerebral venous thrombosis: are there any differences according to presence or absence of cortical vein involvement? Iran J Radiol. 2018; 15:e68250.
4. Lee S, Cho SB, Choi DS, et al. Susceptibility vessel sign for the detection of hyperacute MCA occlusion: evaluation with susceptibility-weighted MR imaging. Investig Magn Reson Imaging. 2016; 20:105–113.
5. Sinnaeve L, Vanopdenbosch L, Paemeleire K. Association of cerebral venous thrombosis and intracranial hypotension: review of 3 cases. J Stroke Cerebrovasc Dis. 2017; 26:e165–e169.
6. Schievink WI, Maya MM. Cerebral venous thrombosis in spontaneous intracranial hypotension. Headache. 2008; 48:1511–1519.
7. Seo H, Choi DS, Shin HS, Cho JM, Koh EH, Son S. Bone subtraction 3D CT venography for the evaluation of cerebral veins and venous sinuses: imaging techniques, normal variations, and pathologic findings. AJR Am J Roentgenol. 2014; 202:W169–W175.
8. Costa P, Del Zotto E, Giossi A, et al. Headache due to spontaneous intracranial hypotension and subsequent cerebral vein thrombosis. Headache. 2012; 52:1592–1596.
9. Yoon KW, Cho MK, Kim YJ, Lee SK. Sinus thrombosis in a patient with intracranial hypotension: a suggested hypothesis of venous stasis. a case report. Interv Neuroradiol. 2011; 17:248–225.
10. Singh R, Cope WP, Zhou Z, De Witt ME, Boockvar JA, Tsiouris AJ. Isolated cortical vein thrombosis: case series. J Neurosurg. 2015; 123:427–433.
11. Zhang D, Wang J, Zhang Q, He F, Hu X. Cerebral venous thrombosis in spontaneous intracranial hypotension: a report on 4 cases and a review of the literature. Headache. 2018; 58:1244–1255.
12. Haacke EM, Mittal S, Wu Z, Neelavalli J, Cheng YC. Susceptibility-weighted imaging: technical aspects and clinical applications, part 1. AJNR Am J Neuroradiol. 2009; 30:19–30.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download