Abstract
Collision tumor is a synchronous neoplasm wherein two histologically distinct tumors co-exist within the same anastomosis site. Collision tumor can occur in any organ, but the incidence is markedly rare. Additionally, preoperative diagnosis can be challenging to the radiologist. Herein, we report an age 60 male with collision tumor of rectal adenocarcinoma and diffuse large B-cell lymphoma, presented as a semi-annular wall thickening and bulky exophytic mass on MR imaging.
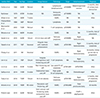
More than 90% of malignant tumors in the colorectum are adenocarcinomas. Malignant lymphomas, conversely, are markedly rare tumors, accounting for only 0.2–0.4% of all malignancy at this site (12). Interestingly, extremely rare collision tumors of these two malignant tumors in the colorectum have been reported. To the best of our knowledge, only 14 cases have been described in the literature (2345678910).
Herein, we report a case of collision tumor of adenocarcinoma and diffuse large B-cell lymphoma involving the rectum with its radiologic findings on magnetic resonance (MR) imaging, with reviewed literature.
An age 62 male was referred to our institution for further evaluation of the rectal mass. The patient had been complaining of anal discomfort persisting for several months. He visited an outpatient clinic and a rectal mass was detected on the hospital's rectal endoscopy. The patient had no medical history, except for diabetes. In the digital rectal examination, a hard-intraluminal mass was palpated in the distal rectum, 30 mm from the anal verge, which strongly suggested rectal cancer. Additionally, the hard mass was fixed to the pelvic side wall. Routine laboratory test results were within normal range. Carcinoembryonic antigen (CEA; normal range, 0–37 u/mL) and carbohydrate antigen 19–9 (CA 19–9; normal range, 0–7 ng/mL) were 15.9 u/mL and 1.2 ng/mL, respectively. He underwent a colonoscopy, abdominal computed tomography (CT) (Fig. 1a), and rectal MR imaging as a baseline study for further investigation of rectal mass. Abdomen CT obtained during the portal venous phase showed an approximately 70 mm large exophytic mass in the left lateral quadrant of the distal rectum. The mass was well margined and revealed homogeneous enhancement without evidence of internal necrosis or hemorrhage. On rectal MR imaging, there was semi-annular wall thickening in the distal rectum, 45 mm from the anal verge and 15 mm from the anorectal junction (Fig. 1b). The lesion extended craniocaudally over 22 mm, from the three to six o'clock direction. Interestingly, an approximately 74 mm sextraluminal mass with a slightly higher signal intensity was compressed and contacted with the intraluminal lesion on oblique T2-weighted axial image (Fig. 1c). The extraluminal mass showed a relatively homogeneous intermediate signal intensity and extended to the left pelvic side wall. Other lesions similar to signal intensity of the extraluminal mass were noted in the seminal vesicle and left femoral head. On diffusion-weighted imaging, we observed that intraluminal and extraluminal lesions were colliding with each other (Fig. 1d). Several enlarged lymph nodes (LNs) were noted in both internal iliac, left external iliac areas, and along superior rectal vessels. All lesions were localized to the pelvis, which showed no evidence of distant lesions on the abdominal CT.
According to the radiological findings, our first impression was a synchronous, double primary tumor in which rectal adenocarcinoma and lymphoma co-exist. A differential diagnosis was rectal adenocarcinoma with seminal vesicle invasion and left femoral head metastasis. It is believed that the enlargement of nodes on the mesorectum and pelvic side-wall is markedly unlikely in the malignant rectal gastrointestinal stromal tumor (GIST).
The patient underwent colonoscopic biopsy for the intraluminal lesion and percutaneous needle biopsy for the extraluminal lesion, respectively (Fig. 1e). The pathologic result was the same as the radiologist's first impression which were moderately differentiated adenocarcinoma and diffuse large B-cell lymphoma (DLBCL) (Fig. 1f–h). First, patient received six-cycle chemotherapy with a regimen of cyclophosphamide, hydroxydaunorubicin, oncovin, prednisone (CHOP) and pegfilgrastim to control the lymphoma. On the following rectal MR imaging, the previously noted lymphoma lesion had disappeared achieving a complete remission. However, a semi-annular wall thickening indicating adenocarcinoma lesion in the distal rectum was noted without interval change (Fig. 2a, b). Subsequent colonoscopy confirmed the existence of residual rectal adenocarcinoma and clinical staging was T3N0M0. The patient received preoperative chemoradiotherapy with capecitabine and 50 Gy/25 fraction, followed by robotic transanal total mesorectal excision with intersphincteric resection and coloanal anastomosis after six-weeks interval. The final histopathologic staging was pT2N0 with cleared circumferential resection margin. Follow-up imaging for a year and a half showed no recurrence.
Collision tumor is a synchronous neoplasm in which two histologically distinct tumors co-exist within the same anastomosis site. Although the terms collision and composite tumor have been used interchangeably, the collision tumor is different from the composite tumor in lacking the histologically intermingling. Approximately 2–7% patients with colorectal carcinoma present with synchronous or metachronous tumor (3). However, collision tumor of rectal adenocarcinoma and DLBCL is markedly rare. To date, only 14 cases of adenocarcinoma and lymphoma in the colorectum have been reported (2345678910) (Table 1). The pathogenesis of collision tumor in terms of carcinogenesis of adenocarcinoma and lymphoma in rectum is controversial. Currently, three hypotheses have been considered in the evolution of collision and composite tumors. The first hypothesis suggests coincidental occurrence rather than any specific association in terms of the marked rarity (6). The second hypothesis is that a common carcinogen may stimulate altering the cellular microenvironment within the same site of which two distinct tumors arise in continuity (6). For example, gastric adenocarcinoma and mucosa associated-lymphoid tissue (MALT) lymphoma may result from an H. pyloric infection (9). The last one suggests that the preceding tumor may have altered the cellular microenvironment or compromised the patient's immune system in advance and then, promoted the development of another tumor (6). Preceding lymphoma leading to defects in the antitumor immunity may induce adenocarcinoma through inactivation of tumor-suppressor genes or activation of oncogenes (3).
On reviewing the reported 14 cases, we describe clinical characteristics. The age of patients was 56–87, and the male to female ratio was 10:3. The location of collision tumor was variable; ileocecal and cecum (n = 5), ascending colon (n = 2), transverse colon (n = 1), sigmoid and rectosigmoid colon (n = 3), and the rectum (n = 3). The most common histologic subtype of lymphoma was B-cell lymphoma (n = 7), follicular lymphoma (n = 2), T-cell lymphoma (n = 2), and etc. (n = 3). Among eight patients with available histopathologic staging of adenocarcinoma, two patients were stage I, two patients were stage II, three patients were in stage III, and one patient was in stage IV. Most patients had symptoms associated with colorectal adenocarcinoma.
The imaging features of the collision tumors of adenocarcinoma and malignant lymphoma derived from 14 reported patients were markedly limited. In preoperative CT, imaging features of the collision tumor were described only in eight patients. Most cases appeared as irregular mass or wall thickening with or without lymphadenopathy (34569). Hence, patients were preoperatively diagnosed with adenocarcinoma. Only one case was misdiagnosed as an abscess (2). Interestingly, none of the patients underwent MRI before surgery. In this case, preoperative rectal MR imaging facilitated detection of the co-existence of adenocarcinoma and lymphoma. Initial baseline CT imaging was limited to the distinguishing features of collision tumors as mentioned above. However, rectal MR imaging could detect different morphology and signal intensity in each tumor. Morphologically, MR imaging can be valuable in differentiating epithelial tumor from subepithelial tumor. Additionally, the homogeneous signal intensity of malignant lymphoma on MR imaging, different signal from co-existing adenocarcinoma, was markedly valuable in the diagnosis. This may be attributed to excellent soft-tissue contrast ratio as well as high spatial resolution of the MR imaging.
From a radiological perspective, it is essential to differentiate this collision tumor from having only adenocarcinoma. Collision tumor may be impossible to accurately diagnosed on biopsy specimen using colonoscopy if both tumors were not sampled. In our case, the anal sphincter may have been sacrificed if the patient was diagnosed with only adenocarcinoma based on the results of a colonoscopy biopsy. Thus, it can be crucial for the radiologist to consider the possibility of collision tumor and to recommend a biopsy in each tumor. In this case, given the appearance of large exophytic mass in the rectum, it is also necessary to consider a malignant GIST as a differential diagnosis. However, raised rolled-edge area in thickened rectal wall showed an acute angle with the adjacent rectal wall. This feature usually suggests an epithelial origin tumor. Additionally, several enlarged LNs, markedly similar in signal intensity to that of the exophytic mass, were valuable in excluding malignant GIST.
In collision tumor of rectal adenocarcinoma and lymphoma, the order of treatment can be a source of debate, because it has not been established which tumor should be treated first. Because of the rarity of collision tumor, evidence-based management based on long-term outcomes is not well defined. In this case, initial CHOP chemotherapy was performed to control lymphoma and then, a complete remission was obtained. Subsequently, low anterior resection was performed following preoperative chemoradiotherapy. As a result, this treatment was markedly successful in avoiding abdominoperineal excision with node incisions on the pelvic side walls. Conversely, Lin et al. (4) reported the patient with collision tumor of stage IV colon cancer and low-grade lymphoma, who received oxaliplatin, 5-FU, and leucovorin first. This was because colon cancer was dominant in that case. Thus, treatment strategy may depend on case by case.
In conclusion, although the collision tumor of rectal adenocarcinoma and DLBCL is markedly rare and seldom diagnosed preoperatively, it can be crucial that the radiologist considers the possibility of collision tumor through detailed MR imaging analysis and recommends biopsy in each tumor.
Figures and Tables
Fig. 1
An age 62 male with collision tumor of rectal adenocarcinoma and DLBCL. (a) Axial CT image obtained from portal venous phase shows a lobulated, relatively homogeneous enhancing mass (arrows) in the left mesorectum. The mass is compressing the distal rectum. (b) On a T2-weighted MR image, the mesorectal mass (asterisk) shows homogeneous, intermediate signal intensity. Additionally, there is a semi-annular wall thickening (arrow) in the left quadrant of the compressed distal rectum, which shows lower signal intensity than that of the mesorectal mass. (c) On a T2-weighted MR image, two nodular lesions, similar to the signal intensity of the mesorectal mass, are noted in the seminal vesicle (arrow) and the left femoral head (arrowheads). (d) On diffusion-weighted image, there is a linear area of non-restricted diffusion (arrows) between the semi-annular wall thickening (obelisk) and the mesorectal mass (asterisk). (e) On colonoscopy, the semi-annular wall thickening on MR image appears as an ulceroinfiltrative lesion with easy contact bleeding.
(f) A photograph of colonoscopic biopsy specimen (Hematoxylin and Eosin [H&E] stanning) shows moderately differentiated adenocarcinoma with glandular formation (arrow). (g) H&E stanning from percutaneous needle biopsy specimen demonstrates an abnormal diffuse infiltrate of small to intermediate-sized lymphoid cells. (h) CD20 B-cell marker are positive.
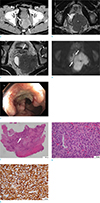
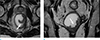
Fig. 2
MR images after six-cycle CHOP chemotherapy. (a, b) On a T2-weighted image, a semi-annular wall thickening (arrows) in the left quadrant of the distal rectum is noted. Previously seen mesorectal mass disappeared and remained as a thin low signal intensity lesion (asterisk), indicating a complete response.
References
1. Korea Central Cancer Registry. National Cancer Center Web site. Accessed November 6, 2019. http://ncc.re.kr.
2. Kus T, Aktas G, Kalender ME, Sari I, Ulker E, Camci C. Collision tumor consisting of primary follicular lymphoma and adenocarcinoma in the cecum: a case report and literature review. Oncol Lett. 2016; 11:2801–2805.
3. Sasaki S, Hatanaka K, Sahara N, et al. Collision tumor of primary malignant lymphoma and adenocarcinoma in the colon: report of a case. Surg Today. 2010; 40:975–981.
4. Lin HH, Jiang JK, Lin JK. Collision tumor of low-grade B-cell lymphoma and adenocarcinoma with tuberculosis in the colon: a case report and literature review. World J Surg Oncol. 2014; 12:147.
5. Lee DY, Hong SW, Chang YG, Lee WY, Lee B, Kang YK. Synchronous T-cell lymphoma in patient with colon cancer: a case report. J Korean Surg Soc. 2012; 83:60–64.
6. Soto AR, Vazquez EG, Grigg-Gutierrez NM, Magno-Pagatzaurtundua P, Caceres W, Toro DH. Conundrum of a large bowel neoplasm: Collision tumor. ACG Case Rep J. 2018; 5:e13.
7. Shigeno T, Fujimori K, Tsuruta F, Nozawa Y, Nagaya T, Maejima T. Ileocecal collision tumor composed of adenocarcinoma and primary malignant lymphoma. Clin J Gastroenterol. 2011; 4:79–84.
8. Mannweiler S, Dinges HP, Beham-Schmid C, Hauser H, Starlinger M, Regauer S. Colliding / concomitant tumors of the intestine: report of 3 cases. Pathol Oncol Res. 2003; 9:188–192.
9. Chang H, Chuang WY, Shih LY, Tang TC. Collision in the colon: concurrent adenocarcinoma and diffuse large B-cell lymphoma in the same tumour. Acta Clin Belg. 2011; 66:302–304.
10. Miyamoto R, Kikuchi K, Uchida A, et al. Collision tumor consisting of a colorectal adenocarcinoma and dissemination of a gastric adenocarcinoma. SAGE Open Med Case Rep. 2018; 6:2050313X17751839.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download